
Article: Fabian Taube, Folke Cerenius, Karl Kristian Faksvåg, Hillary Limo, Amir Khorram-Manesh
Application of two test kits for microbiological water monitoring under harsh conditions – a pilot study
Background: Emergencies confront civilian and military healthcare providers with medical and hygienic challenges due to the lack of potable water. This pilot study aimed to describe the application of two different methods for microbiological monitoring of water in a harsh environment in terms of performance, ease of use, availability, and the possibility of using the results to evaluate water quality.
Methods: Samples from raw water, potable water (water tank) and tap water for consumers were taken from one Swedish and one Norwegian camp, both located within UN Camp Senou, close to Bamako International Airport, and utilizing the same raw water source. The samples were analyzed for the most relevant microorganisms, i.e., heterotrophs, coliforms, Enterobacteriaceae, Enterococci, P. aeruginosa, yeast and mold, by using IDEXX industry-standard methods and a combination of membrane filtration and 3M-Petrifilm.
Results: The IDEXX method used at the Norwegian Camps are easier to utilize and have a broader range of analyzing kits for drinking water assessments.
In addition, IDEXX is better adapted to the requirements of EU-legislation. However, the combination of membrane filtration followed by incubation on 3M-Petrifilm ™, as used at the Swedish camp, is a better field alternative compared to traditional bacteriology, as it eliminates the need to produce and store agar plates.
Conclusions: This pilot study highlights the need for adapted technicalequipment and tools for internal microbiological control of water production in a harsh field environment, common to military deployments, and may facilitate the use of a relatively simple method for water control and ensure the safety of deployed staff in both civilian and military settings.
Keywords: Austere; Healthcare; Microbiological; Military; Water.
Introduction
Public health emergencies, disasters, and armed conflicts are associated with environmental challenges, of which poor water quality and sanitation might constitute the most critical threats to health [1-8]. Since water supply systems, purification, disinfection, and monitoring processes are inferior in poor or disaster-affected nations [7,9-11], deployed staff need to have the knowledge and training about how safe water can be produced and controlled. There are several field methods for water treatment, like heat treatment, ultraviolet light, filtration, and chemical disinfection [8]. The choice of treatment depends on technical availability, environmental impact, organic and microbiological load but also the access to self-monitoring tools, and methods for microbiological monitoring [5,9-11]. In a previous study from Macy et.al 2005 on membrane filtration (MF)/petrifilm™ and IDEXX indicate that both methods could be well suited for field application, given MF, for coliforms and Escherichia coli (E. Coli ) [12]. The results showed consistency between the two methods, but the authors emphasized that accurate results from MF/petrifilm™ might require higher levels of technical skill and quality control than what might be available in laboratories in developing countries. In a 3-year follow-up study on the natural water, with seasonal variation, repeated analysis of coliforms and E. Coli indicated that Colilert DST (Defined Substrate Technology) had a more straightforward laboratory protocol, was quicker to process, and was easier to quantify than MF/petrifilm™ [13].
Using as a comparison, Hörman et. al., evaluated total coliforms, and E. Coli in water samples with IDEXX Colilert 18 and 3M™ Petrifilm™ E. Coli /Coliform Count Plates (Petrifilm™ EC plates) [14-15]. 3M Petrifilm performed worse than Colilert 18 for both positive and negative samples (82.7% vs. 70.6%). Moreover, the Petrifilm™ EC plates provided low sensitivity (39.5-52.5%) but high specificity (90.9- 78.8%). The high specificity of 3M Petrifilm has been verified in other studies as well [16]. In another comparative study, Sartory et. al. suggested that the IDEXX method is an acceptable alternative to the reference membrane method [16-17]. It has the advantage of not requiring confirmation testing and providing confirmed counts within 24 to 28 hours of incubation compared to 40 to 48 hours for the reference membrane method [18]. However, compared to a standard method [19], IDEXX Colilert has been found to indicate higher values for both coliforms and E. Coli , while the opposite was true for Enterolert [20].
In our present study, the application of 3M-Petrifilm™ [21], and IDEXX rapid tests [22], used by the Swedish and Norwegian personnel at two military camps in an environmentally harsh area outside Bamako in Mali, is described in terms of performance, ease of use, availability, and the possibility of using the results to evaluate water quality. Bamako represents a tropical area with large variations in temperature and humidity. In addition, environmental factors such as soil and water pollution might also affect the performance of 3M-Petrifilm™ and IDEXX, as well as the possibility to maintain safe drinking water.
Materials and Methods
Sample sources
The Swedish (SC) and Norwegian (NC) camps utilize the same raw water but use different water purification systems, self-monitoring tools, and methods for microbiological monitoring. At SC, water is purified through reverse osmosis, and with ultraviolet radiation (UV) as a backup. The purified water is then chlorinated to give durability. At NC, water is purified through ultrafiltration followed by chlorination. Three different sampling points were selected at each camp, to be as similar as possible and in line with the definitions of “raw water” “potable water,” and “water for consumers” according to the National Food Agency (NFA) regulations [23]. “Potable water” denotes drinking water from the water tank and “water for consumers” will hereafter be denoted “tap water”.
Sampling procedures
Sampling was performed by adding thiosulphate to chlorinated water sampling bottles according to the ISO standard [28]. The samples were prepared within one hour from the time of sampling. Free chlorine was measured with a HACHDR300 Pocket colorimeter. The samples (n=6) were analyzed using methods and available analysis equipment (Table 1).
Table 1: Overview of the water purification process and control measures.
| Camp | SC (Swedish Camp) | NC (Norwegian Camp) |
| Purification | 1. Reverse osmosis [24] with Blue Box 4000 RO [25]. 2. UV radiation 3. Chlorination (level of free chlorine: 0.2-0.5 mg/l) | 1. Ultrafiltration with Kärcher WTC 5000 UF [26]. 2. Chlorination (level of free chlorine: 1.5-1.7 mg/l). |
| Monitoring | 1. Daily: pH, temperature, turbidity, conductivity, and chlorine. 2. Every two weeks; routine control by microbiological control of raw-, potable- and tap water using MF, followed by incubation on 3M-Petrifilm™ at 22° C (molds) and 36° C (other tests) on a selective medium [21,27]. 3. Yearly, once or twice, in accordance with National Food Agency (NFA regulations); raw-, potable- and tap water are sent to an accredited laboratory for extended microbiological and chemical control [23]. | C 1. Daily: chlorine. 2. Every week: microbiological control of raw, potable, and bottled water, using the IDEXX rapid methods for drinking water analysis, followed by incubation [22]. The samples were quantified by the MPN method (Most Probable Number), which is the estimated number of colonies per ml for heterotrophic plate count (HPC) and the estimated number of colonies per 100 ml sample for the other tests. 3. Yearly, once, or twice; raw-, potable- and tap water are sent to an accredited laboratory for extended microbiological and chemical control. |
Microbiological analysis
The laboratories at SC and NC are air-conditioned. All consumables were stored according to the manufacturer's recommendations. At SC, cultivation was made with 3M Petrifilms™ that contained a water-soluble gelling agent, nutrients for the specific microorganism to be grown, and an indicator that simplifies the reading of the colonies [21]. Quantification was made either by counting the colonies manually or by utilizing plate-reading equipment. 3M Petrifilms™ are intended to analyze bottled water [21, 29]. However, the 3M Petrifilms™ used in this study, i.e., Aqua Coliform Count Plates (AQCC), Yeast and Mold Count Plates (AQYM), Heterotrophic Count Plates (AQHC), and Enterobacteriaceae Count Plates (AQEB) can be used for non-bottled drinking water as well [30].
Prior to preparation, 3M Petrifilms™ were moistened with 1 ml sterile saline. MF was performed according to ISO standard [31] by filtering 100 ml of samples through a 0.45 μm MF-Millipore™ membrane, collecting bacteria and micro-fungi on the filter. The filter was then placed on a moistened 3M Petrifilm™ and incubated as follows:
- For coliforms (AQCC) and Enterobacteriaceae (AQEB): 36° C for 24 hours.
- For heterotrophs (AQHC): 36° C for 48 hours.
- For micro fungal (AQYM): 22° C for 3-5 days.
Analysis of heterotrophs in raw water was made by filtration of 100, 50, and 10 ml of water respectively. No positive or negative controls were included. After incubation, the number of colonies was counted manually. For coliforms, red colonies, i.e. indicative of coliforms, were counted, regardless whether there are signs of flatulence or not (no flatulence is indicative of atypical coliforms). For Enterobacteriaceae, all red colonies with yellow surrounding zone, red colonies with gas bubbles, and red colonies with yellow zone and gas bubbles were counted. For heterotrophs, all red colonies were counted. For yeast and mold, colonies were differentiated and calculated in accordance with the product manual.
At NC, IDEXX Quanti-tray® was used. IDEXX provides a series of test kits to analyze microorganisms in water based on the detection of specific enzymes in different types of indicator organisms [22]. When bacteria containing the specific enzyme grows, the substrate breaks down, and the indicator is released, resulting in a change in color or fluorescence. In this study, Enterolert™, Colilert™, and Pseudalert™ test trays were used.
The results from the samples were compared with each other and against the NFA (National Food Agency)’s regulations on drinking water, as shown below (Table 2) [23].
Table 2. Microbiological reference values for drinking water according to NFA (SLVFS 2001:30).
Blank areas mean that there is no limit value for that parameter.
| Parameters | Potable water | Tap water | ||
Appropriate with remark | unfit for human consumption | Appropriate with remark | unfit for human consumption | |
| Cultivable microorganisms at 22° C | 10 CFU/ml* | 100 CFU/ml | ||
| Intestinal enterococci | Detected in 100 ml | Detected in 100 ml | ||
| Coliform bacteria | Detected in 100 ml | 10 CFU/100 ml | Detected in 100 ml | 10 CFU/100 ml |
| Escherichia coli | Detected in 100 ml | Detected in 100 ml | ||
| Clostridium perfringens | Detected in 100 ml | |||
| Micro fungi | 100 CFU/100 ml | |||
| Actinomycete | 100 CFU/100 ml | |||
* 10 CFU/ ml water applies for disinfected water.
Results
Chemical and physical parameters
At SC, the measured water temperatures at the sampling time were 29.0 (84.2), 28.1 (82.6), and 38.4 (101.1) °C (°F) for raw water, potable water, and tap water respectively. The free, active chlorine concentration was 0.38 and 0.06 mg/l (potable water and tap water respectively). At NC, the corresponding water temperatures were 28.6 (83.5), 29.0 (84.2), and 28.0 (82.4) °C (°F), respectively. The free active chlorine concentration was 1.83 and 1.41 mg/l (potable water and tap water respectively). The high free chlorine values at NC exceeds the recommended value (1.0 mg/l) in the NFA regulations [23] but has been applied in order to compensate for the larger membrane pore size used in the ultrafiltration unit (about 0.02 μm, i.e., that does not guarantee effective filtration of viruses).
The gradient of conductivity throughout the osmotic water purification process measures the filtration capacity and serves as an indirect control of the chemical and microbiological quality of the purified water. Typical conductivity in raw water at SC and NC was in the range of 30-40 mS/m and in tap water 1-2 mS/m.
Microbiological parameters
In Tables 3 and 4, results from samples taken on January 26th and 27th 2021 are presented in CFU (Colony Forming Unit) per 100 ml (heterotrophs; CFU per ml). TNTC (Too-Numerable-To Count) denotes either that the number was too large to count or that the maximum response rate for the test was reached. For raw water, using the MF method (SC), TNTC was reached for all sample volumes (100, 50, and 10 ml) (Figure 1).
Table 3. Result of 3M-Petrifilm™ for six samples taken in January 2021 (RW: Raw Water; PW; Portable Water; TW: Tap Water)
| Camp | SC | NC | ||||
RW | PW | TW | RW | PW | TW | |
| Test volume (ml) | 100 (50/10) | 100 | 100 | 100 (50/10) | 100 | 100 |
Heterotrophs (HPC), | TNTC | 0 | 0 | TNTC | 5 | 20 |
| Coliforms (/100 ml), 36°C (24 h) | 7 | 0 | 0 | 7 | 0 | 0 |
| Enterobacteriaceae (/100 ml), 36° C (24 h) | 4 | 0 | 0 | 6 | 0 | 0 |
Yeast & molds | 35 yeasts | 0 | 0 | 7 yeasts | 2 yeasts | 1 yeast |
Table 4. Result of IDEXX quick test for six samples taken in January 2021. For coliforms, both strong and weak positive wells are counted and added (RW: Raw Water; PW; Portable Water; TW: Tap Water)
| Camp | SC | NC | ||||
RW | PW | TW | RW | PW | TW | |
| Test volume (ml) | 100 (HPC 10 ml) | 100 | 100 | 100 (HPC 10 ml) | 100 | 100 |
Heterotrophs (HPC) | 84 (TNTC) | 0 | 0 | 84 (TNTC) | 0 | 0 |
Coliforms | 5+3 | 0 | 0 | 4+2 | 0 | 0 |
Enterococci | 0 | 0 | 0 | 0 | 0 | 0 |
Pseudomonas | 1 | 0 | 0 | 0 | 0 | 0 |
Heterotrophs in raw water were found to be TNTC, i.e., 84 positive wells (Figure 2 a, SimPlate™), corresponding to > 73.8 CFU/ml, according to the MPN table. Raw water analysis using Enterolert™ gave a “null” result. Analysis using ColilertTM gave a positive signal (yellow color) in six wells from the NC water sample, corresponding to 6.4 CFU/100 ml for coliform bacteria. For raw water at SC, eight wells had a yellow color (Figure 2 b), corresponding to 8.7 CFU/100 ml. Furthermore, fluorescence was not detected, i.e., all samples were negative with respect to E. Coli . Analysis with Pseudalert™ gave one positive well from the raw water sample from SC (Figure 2 c), equivalent to 1.0 CFU/100 ml. All other samples were negative. Although the total number of heterotrophs at 36°C and Pseudomonas are NFA parameters for evaluating bottled water, both are relevant indicators for evaluating tap water and potable water as well.
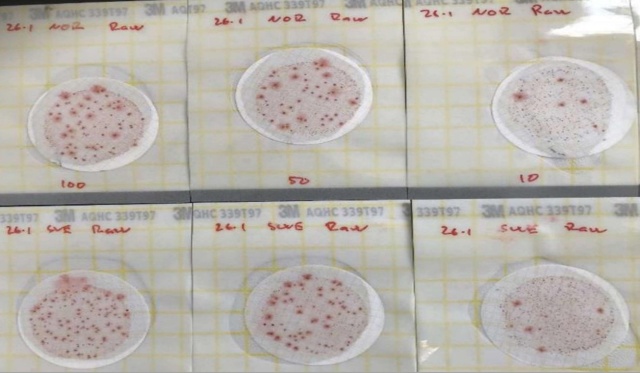 Figure 1: 3M-Petrifilm™ total number of heterotrophs in raw water at SC (lower part of the picture)
Figure 1: 3M-Petrifilm™ total number of heterotrophs in raw water at SC (lower part of the picture)
+ and NC (upper part of the picture) for 100, 50, and 10 ml, respectively.
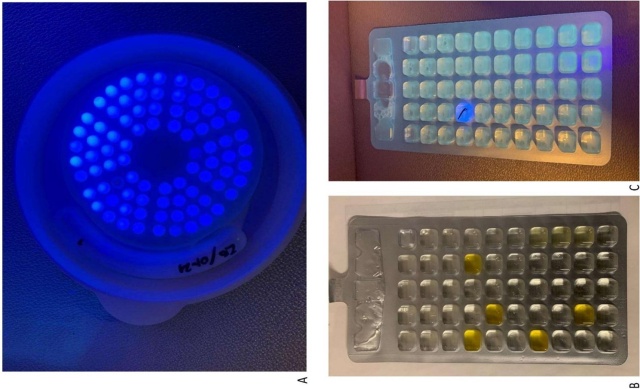
Figure 2: A: SimPlate™ HPC raw water SC, B: ColilertTM raw water SC, C: Pseudalert™ raw water SC.
Temporal variation of coliforms in raw water
Raw water samples are taken from the raw water tank at each camp, both being located close to the joint raw water source. In figure 3, available results of coliforms in raw water samples taken during autumn 2021 at NC (brown line) and SC (blue line) are presented. A routine sampling at the two camps is made independently from each other; however, on one occasion, in week 42, sampling was made on the same date (CFU = 2 at SC, MPN = 0 at NC). As can be seen, both methods detect higher amounts of coliforms during weeks 31-34. In the sample taken during week 34 at NC, 22 of the 84 wells were fluorescent, indicating the presence of E. Coli.
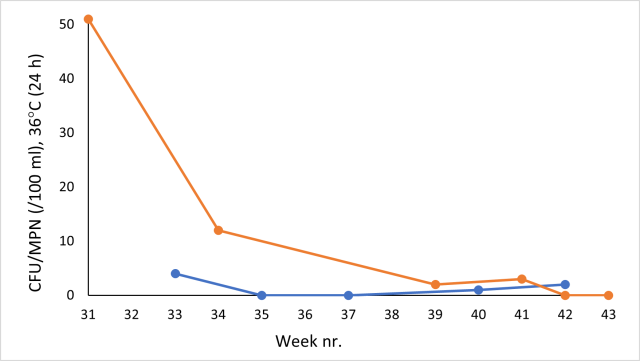 Figure 3: Temporal variation of coliforms in raw water during week 31-43 at NC, using IDEXX (brown line) and SC, using 3M (blue line).
Figure 3: Temporal variation of coliforms in raw water during week 31-43 at NC, using IDEXX (brown line) and SC, using 3M (blue line).
In figure 4, coliforms in raw water during 2021 from NC (brown stacks, 10 samples) and SC (blue stacks, 9 samples) are presented, including a yearly taken reference analysis at SC (grey stack) on February 2nd is included (CFU = 2). For practical purposes, a maximum value of 15 has been set at the y-axis. The actual value on January 24th was 35 CFU, and the actual MPN value on August 3rd was 51.
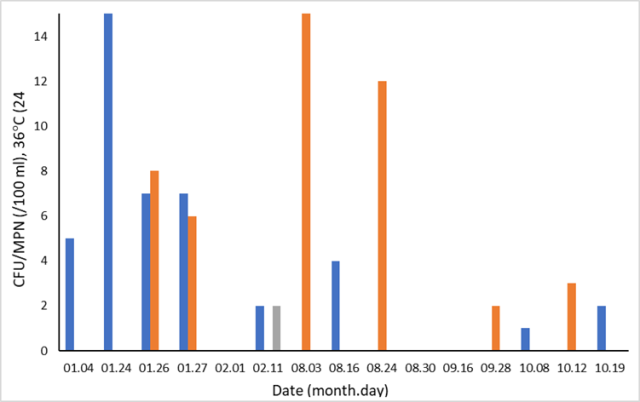 Figure 4: Temporal variation of coliforms in raw water at NC (brown stacks) and SC (blue stacks). A reference sample (grey stack) was taken and analyzed according to DIN EN ISO 9308-1:2017-09 [32]. Dates with no visible stack equals CFU/MPN = 0.
Figure 4: Temporal variation of coliforms in raw water at NC (brown stacks) and SC (blue stacks). A reference sample (grey stack) was taken and analyzed according to DIN EN ISO 9308-1:2017-09 [32]. Dates with no visible stack equals CFU/MPN = 0.
Temporal variation of other microorganisms in raw water
The number of Enterobacteriaceae (blue stack) and yeast/molds (green stack) in raw water samples taken during the rainy season (week 31 35 in figure 5) at SC varied between 0 and 8 CFU (5 samples), and 5-40 CFU (5 samples), respectively, i.e., lower than most of the other weeks during 2021. With a few exceptions, the number of heterotrophs in the samples was TNTC.
The indicator organisms for fecal contamination, i.e., Enterococci (red stack) and Pseudomonas (yellow stack), reached a peak in raw water at NC during weeks 31-34, with MPN = 13-51 and 24-200, respectively. During weeks 39-43, four samples were analyzed with respect to Enterococci and Pseudomonas, all with MPN = 0, except for week 42 (MPN= 1 for Enterococci). With a few exceptions, the number of heterotrophs was TNTC. For practical purposes, a maximum value of 55 has been set at the y-axis. The actual count for Pseudomonas in raw water at NC during week 34 was MPN = 200.
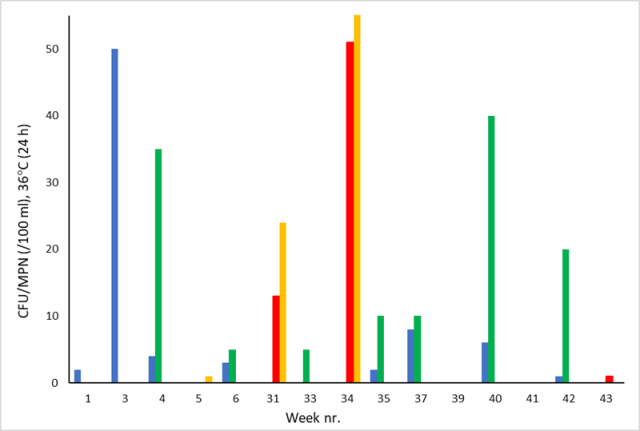 Figure 5: Temporal variation of Enterobacteriaceae (blue stack), yeast/molds (green stack) in raw water at SC and of Enterococci (red stack) and Pseudomonas (yellow stack) in raw water at NC during 2021. Weeks with no visible stack equals CFU/MPN = 0.
Figure 5: Temporal variation of Enterobacteriaceae (blue stack), yeast/molds (green stack) in raw water at SC and of Enterococci (red stack) and Pseudomonas (yellow stack) in raw water at NC during 2021. Weeks with no visible stack equals CFU/MPN = 0.
Discussion
Methods for microbiological monitoring
Two types of indicators could be analyzed with both methods, i.e., heterotrophs and coliforms. In the study conducted by Hörman et al. [15], the authors concluded that Colilert 18 overestimated the total number of coliform bacteria, while Petrifilm EC underestimated the number when compared with the reference method [14]. According to the authors, Colilert is able to induce recovery of injured and stressed coliforms and E. Coli, while methods including MF are known to reduce the recovery of target organisms. In the present study, the agreement regarding coliforms was good both qualitatively and quantitatively, although there was a tendency towards higher counts of coliforms with the IDEXX method. However, due to the low number of samples and large temporal variation of coliforms in raw water (Figures 3 and 4), data are not suitable for statistical evaluation (mean/STDEV = 6,3 /9,9 for 3M-Petrifilm, and 9,1/15,1 for IDEXX).
High bacterial counts were easier to quantify with MF/3M-Petrifilm since it is easy to control the amount of water filtered. However, in raw water samples, the number of heterotrophs was too high to quantify, regardless of the method [21-22]. Hörman et al., found that Petrifilm EC plates has low sensitivity due to small sampling volume, i.e., 1 ml, when inoculated directly on the film [15]. With the reservation of being a small pilot study, our study indicate that the two test-kits has a similar sensitivity when applied in the present way.
In general, IDEXX test kits are easy to use compared to MF and cultivation on 3M-Petrifilm and provide a broader range of analysis kits for drinking water analyses that comply better with the analyses prescribed in NFA regulations [23]. Moreover, sample preparation does not require laboratory experience in the same way as MF, although it requires a UV lamp and equipment for sealing the trays, in addition to sampling trays.
MF/3M-Petrifilm™ resembles traditional bacteriology with outgrown colonies that can be used for further cultivation and analysis but are more feasible and cost-effective since it eliminates the need to produce and store diverse types of agar plates. An additional advantage with 3M-Petrifilm™ over IDEXX is its ability to analyze micro-fungi.
Interpretation of water quality
The dramatic differences between the rain and dry season in Bamako affect the water quality profoundly. Torrential rain on dry, cracked impermeable soil inevitably poses a high risk of penetration of surface water and fecal contamination. The raw water did have a notable variation in coliforms during 2021, and the peak at SC, i.e., 35 CFU on January 24th, did occur in close connection with flooding of the wastewater tank at NC. This indicates that wastewater possibly can penetrate into the Swedish piping system. At NC, E. Coli. as well as pseudomonas and enterococci were detected in raw water during the rainy season, indicating an influence of surface water and fecal contaminants.
High raw water temperatures, from around 28 °C up to 38 °C, imply a potentially favorable environment for microbial growth if nutrient sources are present [4]. Intermediate storage of water in rubber tanks, as applied in both camps, causes an additional risk since rubber of certain qualities can act as substrates for microorganisms, in particular micro-fungi and actinomycetes. Based on our results, there is no indication of "uninhibited" growth of micro-fungi, even though yeast appears to occur.
Analysis of purified water indicated that the water is safe according to the NFA’s regulations on drinking water, although free active chlorine at NC can be above the recommended limits of the NFA regulations [23].
Limitations
The supply chain is uncertain since all consumables must be ordered and transported from within the EU. This limits the possibility to expand sampling procedures, for example with dilution series, in which sterile packaged water, etc., is needed.
Further sampling, including multiple analyses from each sample, is necessary to statistically evaluate the efficiency of the two methods used in this study. However, such analyses are difficult to perform under the prevailing harsh field conditions.
There was no possibility to follow up on the results by sending the samples to an accredited laboratory due to limited refrigeration and cold transportation options.
Conclusions
In this pilot study, we describe the application of two test kits for microbiological monitoring of water in a harsh environment during field operations, with limited laboratory resources and supply chains. An overview of technical performance, the ease of use, and the availability of the methods are presented, together with an interpretation of temporal trends in water quality.
Compared to MF and cultivation on 3M-Petrifilm™, IDEXX analysis kits are easier to utilize, provide a broader range of analyzing kits for drinking water analysis and a has better agreement with the examinations prescribed in the NFA regulations. Although IDEXX is more costly than MF, it might be a better option for some laboratories in developing countries because of the ease of use in the laboratory, or if certain species, such as enterococci, pseudomonas, or E. Coli , are to be analyzed. In addition, in a military environment, especially in a situation with a high rotation of personnel, the user-friendliness of IDEXX will be an advantage. However, the combination of MF followed by incubation on 3M-Petrifilm ™ is a field alternative compared to traditional bacteriology, as it eliminates the need to produce and store agar plates. To obtain a statistically significant evaluation of the techniques used in the present study, repeated sampling must be done.
The rainy season in Bamako, especially in August, creates a high risk of intrusion of surface water and fecal pollution to the raw water. This is a probable explanation for the elevated levels of the indicators for human intervention and fecal contamination detected in this study.
Conflicts of Interest: The authors declare no conflict of interest.
References
- Khorram-Manesh A, Goniewicz K, Burkle FM. Unrecognized risks and challenges of water as a major focus of COVID-19 spread. J Glob Health. 2021; 11: 03016. DOI: 10.7189/jogh.11.03016
- United Nations. Water and Disasters. 2022. [Internet] Available from: https://www.unwater.org/water-facts/disasters/.
- Paturas JL, Smith D, Smith S, Albanese J. Collective response to public health emergencies and large-scale disasters: putting hospitals at the core of community resilience. J Bus Conting Emerg Plan. 2010;4: 286-295.
- World Health Organization. Combating waterborne disease at the household level. [Internet]. World Health Organization 2007. [cited 2007]. Available from: https://apps.who.int/iris/handle/10665/43621.
- Rahman MT, Sobur MA, Islam MS, Ievy S, Hossain MJ, El Zowalaty ME, Rahman AT, Ashour HM. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms. 2020;8(9):1405. https://doi.org/10.3390/microorganisms8091405
- Khorram-Manesh A, Goniewicz K, Burkle FM, Robinson Y. Review of Military Casualties in Modern Conflicts-The Re-emergence of Casualties from Armored Warfare. Mil Med. 2021;usab108. DOI: 10.1093/milmed/usab108.
- Regan PM, Kim H. Water scarcity, climate adaptation, and armed conflict: insights from Africa. Reg Environ Change. 2020;20:129. https://doi.org/10.1007/s10113-020-01713-7
- Ray C, Babbar A, Yoneyama B, Sheild L, Respicio B, Ishii C. Evaluations of low-cost water purification systems for humanitarian assistance and disaster relief (HA/DR). Clean Techn Environ Policy. 2013;15:345-357
- Backer HD, Derlet RW, Hill VR. Wilderness Medical Society Clinical Practice Guidelines for water Disinfection for Wilderness, International Travel, and Austere Situations. Wilderness and Environ Med. 2019;30(4):100-120. DOI: 10.1016/j.wem.2019.06.006
- Darby WM. Medical Risk Assessments: Expanded Mission for SOF Medical Personnel. J Spec Op Med. 2002;2(1):16–19. https://apps.dtic.mil/sti/pdfs/ADA498339.pdf#page=19
- Schoenen D. Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Res. 2020;36:3874-3888
- Macy JT, Dunne EF, Angoran-Benie YH, Kamelan-Tano Y, Kouadio L, Djai KA, Luby SP. Comparison of two methods for evaluating the quality of stored drinking water in Abidjan, Coˆ te d’Ivoire, and review of other comparisons in the literature. J Water Health. 2005;3(3):221-228. DOI: 10.2166/wh.2005.042
- Buckalew DW, Hartman LJ, Grimsley GA, Martin AE, Register KM. A long-term study comparing membrane filtration with Colilertw defined substrates in detecting fecal coliforms and Escherichia coli in natural waters. J Environ Manage. 2006;80(3):191-197. DOI: 10.1016/j.jenvman.2005.08.024
- International Organization for Standardization, ISO 9308-1:2014, Water quality — Enumeration of Escherichia coli and coliform bacteria — Part 1: Membrane filtration method for waters with low bacterial background flora. [Internet]. [cited 2019]. Available from: https://www.iso.org/standard/55832.html
- Hörman A, Hänninen ML. Evaluation of the lactose Tergitol-7, m-Endo LES, Coliert 18, Readycult Coliform 100, Water.Check-100, 3M Petrifilm EG, and DryCult Coliform test methods for detection of total coliforms and Escherichia coli in water samples. Water Res. 2006;40(3):3249-3256. DOI: 10.1016/j.watres.2006.06.024
- Schraft H, Watterworth LA. Enumeration of heterotrophs, fecal coliforms, and Escherichia coli in water: comparison of 3Mk Petrifilmk plates with standard plating procedures. J Microbiol Methods. 2005; 60(3):335-342. DOI: 10.1016/j.mimet.2004.10.008
- International Organization for Standardization, ISO 16266–2:2018. Water quality — Detection and enumeration of Pseudomonas aeruginosa — Part 2: Most probable number method. [Internet]. [cited 2018]. Available online: https://www.iso.org/obp/ui/#iso:std:iso:16266:-2:ed-1:v1:en.
- Sartory DP, Brewer M, Beswick A, Steggles D. Evaluation of the Pseudalert/Quanti-Tray MPN Test for the Rapid Enumeration of Pseudomonas aeruginosa in Swimming Pool and Spa Pool Waters. Curr Microbiol. 2015;71(6):699-705. DOI: 10.1007/s00284-015-0905-8.
- International Organization for Standardization, ISO 9308-2:2012. Water quality — Enumeration of Escherichia coli and coliform bacteria — Part 2: Most probable number method. [Internet]. [cited 2018]. Available online: https://www.iso.org/standard/70091.html.
- Valente MS, Pedro P, Alonso C, Borrego JJ, Dionısio L. Are the defined substrate-based methods adequate to determine the microbiological quality of natural recreational waters? J Water Health. 2010;8(1):11-19. DOI: 10.2166/wh.2009.220
- 3M Petrifilm™. Aqua Plates for Water Testing. [Internet]. [cited 2022]. Available from: https://www.3m.com/3M/en_US/p/d/v000207897/.
- IDEXX.com. IDEXX Water testing solutions. [Internet]. [cited 2022]. Available from: https://www.idexx.com/en/water/.
- Swedish National Food Agency. Regulation on potable water, SLVFS 2001:30. (Sweden's national implementation of Council Directive 98/83/EC on the quality of drinking water). [Internet]. [cited 2021]. Available from: https://www.livsmedelsverket.se/om-oss/lagstiftning1/gallande-lagstiftning/slvfs-200130.
- International Organization for Standardization, ISO 23446:2021. Marine technology — Product water quality of seawater reverse osmosis (RO) desalination — Guidelines for municipal water supply. [Internet]. [cited 2021]. Available from: https://www.iso.org/standard/75607.html.
- MuchMoreWater. We deliver water. [Internet]. [cited 2022]. Available online: http://www.muchmorewater.com/.
- Kärcher. Water Treatment. [Internet]. [cited 2022]. Available from: https://www.kaercher.com/int/professional/cleaning-and-care-products/professional/water/water-treatment.html.
- European Pharmacopoeia. Chapter 2.6.12. Total viable aerobic count. (pp 155). [Internet]. [cited 2005]. Available from: http://uspbpep.com/ep50/2.6.12.%20Microbiological%20examination%20of%20non-sterile%20products%20(total%20viable%20aerobic%20count).pdf.
- International Organization for Standardization, ISO 19458:2006. Water quality — sampling for microbiological analysis. [internet]. [cited 2020]. Available from: https://www.iso.org/standard/33845.html.
- 3M. Food Safety. When it comes to water testing. [internet]. [cited 2012]. Available from: https://iwab.se/50.0.2.0/522/download_1339.php.
- Swedish supplier of 3M-Petrifilm, Triolab (personal communication, 2022).
- International Organization for Standardization, ISO 8199:2018. Water quality — General requirements and guidance for microbiological examinations by culture. [internet]. [cited 2018]. Available from: https://www.iso.org/standard/64151.html.
- Water quality - Enumeration of Escherichia coli and coliform bacteria - Part 1: Membrane filtration method for waters with low bacterial background flora (ISO 9308-1:2014 + Amd 1:2016); EN ISO 9308-1:2014 + A1:2017. [internet]. [cited 2017]. Available from: https://www.beuth.de/de/norm/din-en-iso-9308-1/269260285.
Authors:
Fabian Taube 1, 2 *
Folke Cerenius 1
Karl Kristian Faksvåg 3
Hillary Limo 4
Amir Khorram-Manesh 1,5,6
1 Department of Research and Development, Swedish Armed Forces Center for Defense Medicine, 426 76 Västra Frölunda, Gothenburg, Sweden; [email protected]
2 Institute of Medicine, Sahlgrenska Academy, Gothenburg University, 41345, Gothenburg, Sweden. [email protected]
3 Forsvarets Sanitet, Norwegian Armed Forces Joint Medical Services, 2058 Sessvollmoen , Norway; [email protected]
4 Valiant Integrated Services, Bole Sema 93, Logements, Bamako, Mali 09896; [email protected]
5 Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, Gothenburg University, 413 90, Gothenburg, Sweden
6 Gothenburg Emergency Medicine Research Group (GEMREG), Sahlgrenska Academy, Gothenburg University, 413 45, Gothenburg, Sweden
Date: 09/02/2022
Source: Fabian Taube, Folke Cerenius, Karl Kristian Faksvåg, Hillary Limo, Amir Khorram-Manesh











