
Article
Q Fever – A Differential Diagnosis of Operational Relevance
Department of Trauma Surgery and Orthopaedics, Septic-reconstructive Surgery, Sports Traumatology1 (Medical Director: Colonel (MC) Prof Dr B. Friemert) of the Bundeswehr Hospital Ulm (Hospital Commander: Dr A Kalinowski), Department of Anaesthesiology, Intensive Care and Emergency Medicine2 (Medical Director: Captain (MC) M. Benker) of the Bundeswehr Hospital Berlin (Hospital Commander: Commodore (MC) Dr K. Reuther), Department of Diagnostic and Interventional Radiology3 (Medical Director: Colonel (MC) Dr S. Waldeck) of the Bundeswehr Central Hospital Koblenz (Hospital Commander: Brigadier (MC) Dr J. Brandenstein), Medical Clinic Cochem4 (Director: Colonel (MC) Dr T. Emser), and Department of Internal Medicine5 (Medical Director: Colonel (MC) Dr C. Busch) of the Bundeswehr Hospital Hamburg (Hospital Commander: Brigadier (MC) Dr J. Hoitz)
Summary
Background: Coxiella burnetii is a zoonotic and obligatory intracellular pathogen commonly present in operational areas of the Bundeswehr. Transmitted from animals, the gram negative bacterium can cause a highly feverish infectious disease which often leads to pneumonia.
Case report: A 42 years old patient presented at the German “Major MC Dr. Thomas Broer”[2] field hospital in Mazar-e-Sharif (Afghanistan) complaining of fever with chills and severe malaise. Chest x-ray showed a left sided pneumonia, pathogen proof was not possible at this early stage. Empirical initial antibiotic therapy was not successful. After treatment with doxycycline the patients’ symptoms improved and the patient recovered. Finally, serological examination and PCR proved an infection with Coxiella burnetii: The patient had been suffering from Q-fever.
Conclusions: Q-fever caused by an infection with Coxiella burnetii has to be taken into account as a potentially differential diagnosis in cases of feverish illness especially on deployment in endemic areas. In most cases the infection shows an asymptomatic pattern, however, pneumonia, endocarditis and neurological effects can occur. Especially, a chronic disease must be feared. After serological and PCR diagnostics antibiotic therapy, with Doxycyclin e. g., is obligatory.
Keywords
Q-fever, pneumonia, fever of unknown origin, Coxiella burnetii, pulmonary infiltrations, infectious disease, missions abroad
Patient history
Upon admission, the patient complained of fever that had lasted for about one week already and had initially been treated symptomatically by the unit physician who suspected a common cold. In the course of the disease, however, the patient's general condition had deteriorated dramatically; he was now suffering from chills, unproductive (dry) cough, and pronounced fatigue. In addition, respiratory chest pain on the left side had developed, in particular when taking deep breath. His temperature had continuously been elevated; he had not noticed a particular rhythm.
Six weeks before his current presentation, the patient had been in Sudan for ten days without malaria prophylaxis. He had been travelling through rural areas with free livestock farming but had had no direct contact with animals. He had been undertaking similar journeys for 5 years already, always without medication prophylaxis and diseases. He had received all necessary vaccinations, including vaccination against yellow fever; since his last relationship 6 months ago, he has had no high-risk sexual contacts. The patient did not have previously existing conditions, was not on long-term medication and had no known allergies.
Findings on admission and initial diagnosis
On admission, the patient was fully oriented but was sweating with an obviously poor general state; physical findings: heart rate 60/min, blood pressure 120/56 mmHg, SpO2 93%, temperature: 39.8° C, respiration rate 23/min using the auxiliary respiratory muscles. The patient showed bilateral vesicular breathing sounds, his heart sounds were rhythmic and clear without pathologic heart murmur. Abdominal findings were normal, there were no peripheral oedemas; peripheral pulses were palpated. Arterial blood gas analysis (ABG): pH value 7.6, pCO2 24 mmHg, pO2 60mmHg, SO2 97%. Abdominal ultrasonography was normal, ECG appropriate to age. Laboratory findings: Leukocytes 6.5/nl (normal range: 4–10/nl), CRP 18 mg/dl (normal range: <0.5 mg/dl), PCT 0.25 ng/ml (normal range: <0.5 ng/ml), Quick's value 52 % (normal range: 70–130 %); urine tested positive for protein and glucose.
To complete initial diagnostics, blood cultures and blood samples for serological examination were taken during a fever attack. The lung X-ray (Fig. 1) revealed a left-sided retrocardiac infiltrate with a minimum pleural effusion.
Working diagnosis
Taking into consideration clinical, radiological and laboratory findings resulted in the suspected diagnosis of "moderate pneumonia". In accordance with guidelines, empirical therapy with cefuroxime and clarithromycin was initiated; the latter to cover pathogens of atypical pneumonia [16]. The patient was hospitalized for further treatment.
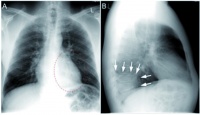 Abb. 1: Röntgenbild der Lunge: (A) Thorax p.a.: gestrichelte Linie markiert Infiltrat (B) links retrokardiales, pneumonisches Infiltrat, durch weiße Pfeile markiert
Abb. 1: Röntgenbild der Lunge: (A) Thorax p.a.: gestrichelte Linie markiert Infiltrat (B) links retrokardiales, pneumonisches Infiltrat, durch weiße Pfeile markiert
Course
Administration of additional antipyretic medication initially resulted in improvement of the general condition. But the patient continued to have a high need for fluids before producing sufficient urine output. An infection by most of the causative agents of atypical pneumonia was excluded by first microbiological tests (urine sample to exclude legionella, blood sample to exclude mycoplasma infection), so administration of clarithromycin was discontinued after one day. It was remarkable at this point that the patient did neither react with leukocytosis nor with an increase of procalcitonin (PCT) to the disease but that increased CRP was the only inflammation parameter.
Initially, the more frequent causative agents of pneumonia could not be detected in previous microbiological tests (table 1). Given the unclear clinical picture, cefuroxime administration was discontinued on the second day to critically evaluate the further course of the disease. Because of the travelling history, threefold assessment was made (thick and thin blood smears and rapid diagnostic test) for malaria which was ruled out after negative results.
After completion of the antibiotic treatment, the general condition of the patient deteriorated. For two days, the patient experienced increasing temperature, most intense, therapy-resistant headaches and dyspnoea; peripheral oedemas occurred with normal retention parameters. CRP, and meanwhile also PCT (0.85 ng/ml, normal range: <0.5 ng/ml), were increasing; the patient suffered from lymphocytopenia. The patient, an otherwise healthy man, refused to undergo HIV test to exclude other potential differential diagnoses.
Because of the progressing clinical findings, a cranial CT scan without intravenous contrast material contrast was performed (problem: infection, inflammation, cerebral haemorrhage, increased intracranial pressure) and of the thorax (problem: infiltrations) (fig. 2). Intracranial findings were normal; in particular, there was no cerebral oedema, no indication of increased intracranial pressure or infection.
The CT scan of the thorax showed several pathologic changes. Segment pneumonia was identified in segment 10 of the left lung, as well as atypical infiltrates in the remaining left inferior lobe and in the right superior and inferior lobes. The upper abdominal organs that were also scanned were normal. Lumbar puncture excluded infection of the central nervous system.
Due to diagnostic procedures and the clinical course, a "bacterial pneumonia" had to be assumed, still. Up to this point in time (day 4 after admission), no pathogen had been identified with the result that a broad antibiotic therapy had to be started empirically that had to cover also pathogens like Salmonella typhi and intracellular pathogens like Coxiella and Rickettsia. Intravenous administration of ceftriaxone and doxycycline was started, respiratory training was intensified.
The new treatment
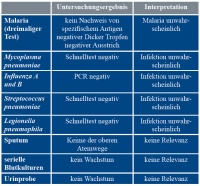 Table 1: Pathogen search in the country of deployment
Table 1: Pathogen search in the country of deployment
resulted in a quick amelioration of the clinical symptoms, including easing headaches, dyspnoea, and decreasing body temperature; during the following seven days, inflammation parameters successively fell to normal ranges. Antipyretic medication and intravenous administration of fluids could be terminated; doxycycline was switched to oral medication after five days. Peripheral oedemas were recessive. After 8 days, administration of ceftriaxone was terminated; the patient was to continue to take the oral doxycycline until day 14. After the intravenous medication had been terminated, the patient was discharged in good general condition after altogether 13 days. He subsequently returned to the Netherlands.
Confirmed diagnosis
A few days after his discharge, the final microbiological findings arrived from Germany (table 2): There had been an acute infection with Coxiella burnetii (table 3). Diagnosis: acute Q fever.
Discussion
Coxiella burnetii
Coxiella burnetii is a zoonotic and obligatory intracellular pathogen that occurs worldwide. The gram negative bacterium is transmitted from animals, e.g. sheep, goats, and cattle. It is transmitted through direct contact with animals, by dust aerosols, droplet infection, transcutaneously, and through consumption of raw milk products. Already 1 to 10 pathogens are sufficient to cause infection [1, 2, 15]. The bacterium is extremely unlikely to spread by person-to-person contact; nosocomial infections have not been described. Due to its high contagiousness, the pathogen is assigned to risk group 3. In the past, C. burnetii had been part of the biological weapons programmes of the United States and the former Soviet Union [1, 3].
Coxiellas are ingested and phagocytized by monocytes and macrophages; due to the low pH value in the phagolysosomes, they are metabolically active and multiply (duplication period: 20–45 h). They occur in different forms; in mammal cells, they above all grow as "large cell variant" (LCV) and form environment resistant, spore-like particles. Two antigen forms develop which matter for antibody response and, thus, for the serological proof (phase I and II) [1, 2].
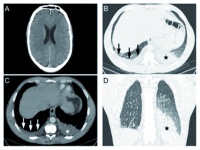 Fig. 2: cranial CT and chest CT, 4 days after chest X-ray: normal intracranial findings; exemplary slice; infiltrate segment 10 left inferior lobe (asterisk) and right-sided pleural effusion (arrows); finding corresponding with image B in different windowing; infiltrate segment 10 left inferior lobe in the frontal plane (asterisk)
Fig. 2: cranial CT and chest CT, 4 days after chest X-ray: normal intracranial findings; exemplary slice; infiltrate segment 10 left inferior lobe (asterisk) and right-sided pleural effusion (arrows); finding corresponding with image B in different windowing; infiltrate segment 10 left inferior lobe in the frontal plane (asterisk)
Q fever
Infection with C. burnetii leads to febrile illness, the so-called Q fever. As to its course, it can be subdivided into an acute and a chronic form as well as to its places of manifestation. Incidence in Germany is 1–5 / 1,000,000 inhabitants; there are occasional outbreaks in sheep flocks [1].
Clinical course
After an incubation period of 3–30 days, only 5–20% of the cases develop symptomatic medical conditions with fever, attacks of sweating, gastrointestinal complaints, as well as a marked sense of fatigue [1, 4, 5]. Also striking are most intense headaches that do not react to analgetics. In most cases the disease is self-limiting; in up to 2% of the cases, however, pneumonia develops, often looking like a lobar or atypical pneumonia. Hepatitis may be an associated finding and can be identified by increased transaminases. Meningitis and encephalitis may also occur; in which case mononuclear cells and an increased content of protein are found in the cerebrospinal fluid (CSF).
Complications
In 5–15% of the patients the disease takes a chronic course during which the above symptoms can become chronic and endocarditis may occur. 20–30% of the patients develop a chronic fatigue syndrome [1, 6]. In case of symptoms, lethality is 2% [1, 7].
Diagnosis
Serological tests and PCR analysis to detect C. burnetii DNA are employed for diagnosis. The indirect immunofluorescence test (IFT) is the standard reference method and will yield positive test results about 1–2 weeks after the onset of the disease. Detection of antibodies can also be accomplished by means of ELISA or Western blot. Given the diagnostic gap of 1–3 weeks until antibody production (at first, antibodies directed against the phase 2 antigen are produced) detection of C. burnetii DNA in the blood by means of PCR analysis increasingly takes hold [1, 2, 5, 6]. In principle, cell culture is possible using special media; due to the reasons above, it is only of secondary importance.
Duty to Report
In the event of a direct or indirect detection of a suspected or known acute infection with C. burnetii, notification of the Public Health Office is obligatory in accordance with German legislation (Section 7, Paragraph 1 of the Infection Protection Act).
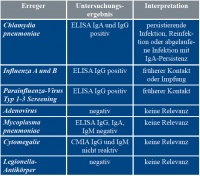 Table 2: Serological test results
Table 2: Serological test results
Treatment
In case of clinical symptoms, doxycycline is the first-line therapy against Q fever. Dosage should be 100 mg b.i.d. for 14 days. In case of pregnancy or other contraindications for doxycycline, cotrimoxazole 160/800 mg b.i.d can be administered as second-line therapy [1, 2, 6, 7, 9]. The latter can also be used during pregnancy or for children under eight years of age in weight-adapted doses [2]. In case of a chronic infection, a combination of doxycycline and hydroxychloroquine must be administered for at least 18 months for alkalisation [1, 2, 10]. Other alternative antibiotics for treatment are macrolides, like azithromycin and clarithromycin, fluoroquinolones or tigecycline [1, 2].
Course and prognosis
In most cases, infection with C. burnetii takes an asymptomatic course. In the event of confirmed acute infection, however, antibiotic treatment should be initiated. On the one hand, when treated, symptoms of the disease and fever remit after 2–3 days on average, whereas without treatment easing of symptoms may be expected only after 12.5 days on average [2]. On the other hand, there are indications that early treatment can prevent complications and a chronic course (risk approx. 2–15%) including for instance endocarditis or vascular infection [1, 3, 6]. We must, in particular, prevent development of the Q fever fatigue syndrome which, if left untreated, in 20–30% of the patients brings about a long-term reduction in activity and, thus, reduced operational readiness of soldiers [4, 6].
Case Discussion
The above case report describes a patient with high fever caused by pneumonia. A definitive diagnosis could be made only after C. burnetii had been detected in a blood sample. At that point in time, however, the patient had already been discharged in a largely better condition after empiric antibiotic treatment that was also effective against Coxiella.
The patient's pneumonia was assessed to be moderate with the result that in accordance with the German S3 Clinical Practice Guideline "Management of adult community-acquired pneumonia and prevention" – as of Feb 2016 – [16] initially the combination of a second-generation cephalosporin (cefuroxime) and a macrolide (clarithromycin) was selected. The initial combination with clarithromycin was only moderately effective against C. burnetii. Macrolides are still not first-line antibiotics for the treatment of Q fever [1, 2].
In retrospect, the symptoms described perfectly matched the diagnosis of "Q fever". High fever, extreme fatigue, therapy-resistant headaches, segment pneumonia, and in addition atypical infiltrates match full-blown Q fever, with only 2% of the infected, however, developing these manifestations [1].
Retrospectively, the route of infection cannot be traced back. The stay in Sudan six weeks before was significantly beyond the maximum incubation period of up to 30 days and, thus, is ruled out as potential source of infection. As security officer in a consulate, however, the patient also in Afghanistan was in contact with potential vector animals [4].
Since pneumonia is frequently diagnosed, infection with C. burnetii cannot routinely be expected in the first place. Only if the medical condition persists or initial antibiotic treatment fails, the differential diagnosis spectrum should be extended, as was done here. Initializing and monitoring antibiotic treatment, here, should not be guided by laboratory findings alone; in the end, it is the clinical impression that matters.
There is a diagnostic gap, especially at the onset of disease, because it takes 1–2 weeks until antibodies are produced which can then be detected by serologic tests. This is why DNA detection by means of PCR analysis has gained in importance in recent years, since it enables earlier confirmation of the diagnosis [2, 5, 6]. In the case described, antibodies were detected by means of the indirect immunofluorescence test (IFT) which was certainly due to the protracted course of disease before the patient was admitted to the clinic.
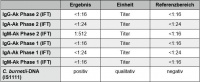 Table 3: C. burnetii diagnostic procedure, acute infection
Table 3: C. burnetii diagnostic procedure, acute infection
Relevance for military medicine
For decades now, German soldiers have been on deployments around the world and they regularly get in touch with pathogens and resistance patterns that are rather rare in their home country [3, 9, 11, 12]. The major conflicts of the last centuries have time and again shown how severely operational readiness of own forces can be diminished by infectious diseases [4]. Although continuous efforts including intense prophylaxis and preventive measures and more effective therapies have reduced the threat to the soldiers, diseases occur again and again which are transmitted by a great number of viral, parasitic, and bacterial pathogens. Often these are diseases that only rarely occur in Germany. So the first step to "think about it!" rates high.
Because of the chains of transmission and environmental resistance, Coxiellas occur in the countries of deployment, like Kosovo or Afghanistan, and may constitute a health hazard [1]. Just in April 2016, there was an outbreak of Q fever in Kosovo during which 50 persons fell ill. In 35 soldiers pneumonia was radiologically confirmed, one soldier died; this is in line with the above mortality rate [15]. Data from Afghanistan are also available. In 1.7% of the British soldiers deployed in Helmand province in Afghanistan seroconversion as indication for infection with C. burnetii was proved [4]. US-Americans report significantly higher results of approx. 10% as to seroconversion from their experience in Iraq [13].
Way Ahead
In its microbiological laboratory, the field hospital in Mazar-e-Sharif has available a sound spectrum of methods to detect pathogens by means of cell culture, rapid tests, or PCR [1, 2, 14]. Diagnostic tests to prove C. burnetii cannot be carried out in the country of deployment, yet; the sample material must be sent to the Central Institute of the Bundeswehr Medical Service Koblenz for analysis. Due to the logistic effort required, this leads to a time delay in addition to the above diagnostic gap. Given the British and US-American data on incidence and prevalence as well as our own experience in Kosovo, it should be considered to also establish PCR diagnosis capability for C. burnetii in the country of deployment in the future.
Key statements / conclusion
- During operations abroad, modified spectrums of – sometimes rare – pathogens are encountered the diagnosis of which should be known.
- Appropriate clinical findings provided, also intracellular pathogens should be considered in the event of pneumonia.
- As a notifiable disease, Q fever is a differential diagnosis in the event of pulmonary infiltrations that must be considered, in particular during deployments abroad.
- Proof of acute Q fever infection is given through antibody detection and PCR analysis for C. burnetii DNA.
- Q fever requires antibiotic treatment, primarily with doxycycline, for pregnant women and children under eight years of age with cotrimoxazole (in weight-adapted doses).
References
- Coxiella burnetii- Erreger des Q(query)-Fiebers [Causative agent of Q fever]; Bundesgesundheitsbl [Federal Health Gazette] 2013;56: 1178–1190
- Kersh GJ:, Antimicrobial therapies for Q fever. Expert Rev Anti Infect Ther 2013;11: 1207–1214.
- Frangoulidis D: , Einsatz molekularer Typisierungsmethoden zur Untersuchung von Q-Fieberausbrüchen. [Application of molecular typing methods to examine Q fever outbreaks.] Wehrmedizinische Monatsschrift 2012; 56(4): 90–95.
- Newman ENC, Johnstone P, Bridge H et al.: Seroconversion for infectious pathogens among UK military personnel deployed to Afghanistan 2008–2011., Emerg Infect Dis 2014; 20: 2015–2021.
- Hagenaars JCJP, Renders NH, van Petersen AS et al.: Serological follow-up in patients with aorto-iliac disease and evidence of Q fever infection. Eur J Clin Microbiol Infect Dis 2014; 33: 1407–1414.
- Keijmel SP, Krijger E, Delsing CE et al.: Differentiation of acute Q fever from other infections inpatients presenting to hospitals, the Netherlands. Emerg Infect Dis 2015; 21: 1348–1356.
- Shishido AA, Letiaia AG, Hartzell JD: Q Fever. US Army Med Dep J. 2016 Jan-Mar: 68–70.
- Omsland A, Hackstadt T, Heinzen RA:, Bringing culture to the uncultured: Coxiella burnetii and lessons for obligate intracellular bacterial pathogens. PLOS Pathog 2013; 9(9): e1003540
- Oltmanns K: Luftnot und Lungeninfiltrat im Auslandseinsatz- Immer eine Infektion? [Shortness of breath and pulmonary infiltrate on deployments abroad – always an infection?], Wehrmedizin und Wehrpharmazie 2011; 3: 25–28.
- Kampschreur LM, Wegdeam-Blans, MCA, Wever PC et al.: Chronic Q fever diagnosis – consensus guideline versus expert opinion. Emerg Infect Dis 2015; 21: 1183–1188.
- Frickmann H, Hagen RM: Bakterielle Erreger mit atypischen Resistenzmustern- ein Update. [Bacterial pathogens with atypical resistance patterns – an update.] Wehrmedizinische Monatsschrift 2012; 56(10): 234–239.
- H. Frickmann H, Hinz R, Ebert KP et al.: Atypisch resistente Erreger in subtropischen und tropischen Einsatzgebieten und bei Kriegsversehrten aus Krisengebieten- ein Update. [Atypically resistant pathogens in subtropical and tropical theatres and in war-disabled persons from crisis areas – an update.] Wehrmedizinische Monatsschrift 2015; 59(3): 81–86.
- Anderson AD, Baker TR, Littrell AC et al.: Seroepidemiologic survey for Coxiella burnetii among hospitalized US troops deployed to Iraq. Zoonoses Public Health 2011; 58: 276–283.
- Angelakis E, Mediannikov O, Stein A, Bassene H Sokhna C, Roault D: Throat swab samples for diagnosis of Q-fever, Am J.Trop Med Hyg 2014; 90(1): 147–148.
- Fengler I: Hochmobile Laborfähigkeiten im Einsatz - Rapidly Deployable Outbreak Investigation Team (RDOIT) zur Aufklärung des Q-Fieberausbruchs im Kosovo im April 2016. [Highly mobile laboratory capabilities on deployments – Rapidly Deployable Outbreak Investigation Team (RDOIT) investigates Q fever outbreak in Kosovo in April 2016.] Wehrmedizinische Monatsschrift 2016; 60(9/10): 289
- Ewin S et al.: S3-Leitlinie: Behandlung von erwachsenen Patienten mit ambulant erworbener Pneumonie und Prävention – Update 2016 vom 21.01.2016; [S3 Clinical Practice Guideline: Management of adult community-acquired pneumonia and prevention – 2016 update of 21 Jan 2016] http://www.awmf.org/uploads/tx_szleitlinien/020-020l_S3_ambulant_erworbene_Pneumonie_Behandlung_Praevention_2016-02-2.pdf (accessed: 27 Sep 2016)
Manuscript information
Submitted: 29 June 2016
Revised version accepted: 2 October 2016
Referencing
German:
Zischek C, Fürstenberg M, Schneider T, Giebel S, Engels M: Q-Fieber- eine Differentialdiagnose von Einsatzrelevanz. Wehrmedizinische Monatsschrift 2016; 60(11): XXX – YYY
English:
Zischek C, Fürstenberg M, Schneider T, Giebel S, Engels M: Q-fever- a differential diagnosis relevant for military missions. Wehrmedizinische Monatsschrift 2016; 60(11): 327 - 332
For the Authors
Major, Medical Corps, Dr Christoph Zischek
Department of Trauma Surgery and Orthopaedics, Septic-reconstructive Surgery, Sports Traumatology
Bundeswehr Hospital Ulm
Oberer Eselsberg 40, 89081 Ulm
Email: [email protected]
Eine Deutsche Version des Textes finden Sie hier.
A german version of this article you will find here.
[1] Das Deutsche Einsatzlazarett Mazar-e-Sharif trägt den Namen des am 15. April 2010 gefallenen Sanitätsstabsoffiziers
[2] The German field hospital in Masar-e-Sharif is named by Major Dr. Thomas Broer; he was a physician who was killed in action on 15 April 2010 in Afghanistan
Date: 12/09/2016
Source: Wehrmedizinische Monatsschrift 2016/11











