
Article
Bacillus cereus contamination in coffee makers at several Bundeswehr dining and recreational facilities
Laboratory Department II, Veterinary Medicine, Mainz (headed by Colonel (VC) Dr. Th. Reiche), Central Institute of the Bundeswehr Medical Service, Koblenz (headed by Colonel (MC) Prof. Dr. D. Leyk)
Introduction: Milk added to mixed beverages from fully automatic coffee makers carries a risk of bacterial contamination, in particular with toxigenic Bacillus (B.) cereus. To assess the risk to consumers, information about the occurrence of this pathogen in coffee makers is vital.
Materials and Methods: Over a period of two months, samples of the milk content in ready-to-drink hot beverages and the topping powder used in the machine as well as the original packaging of the topping of that same charge were taken from 22 coffee makers used in 21 Bundeswehr dining and recreational facilities and examined for the presence of B. cereus. Microbiological examination of the samples included the detection of B. cereus colonies in culture and their ability to produce Nhe and Hbl enterotoxins. The samples were also examined for the presence of these bacterial toxins.
Results: B. cereus was found in all three sample types at concentrations between 101 to 106 CFU/g. The isolated germs were able to produce at least one of the two enterotoxins and four of the samples contained at least one of the two enterotoxins. In recreational facilities the prepared drinks were contaminated with B. cereus, whereas in dining halls the germ was detected only in the original packaging or topping samples taken from the coffee machines.
Discussion/Conclusions: The test results suggest that, owing to its great resilience and ability to produce a biofilm, B. cereus is present not only in foods but also in kitchen equipment that comes into contact with these foods. Further tests must be conducted to clarify the extent to which this poses an actual risk to the health of consumers. In any case, any risk analysis should consider coffee makers in dining halls and recreational facilities as a possible source in case of an outbreak of foodborne disease. Cleaning and disinfection with effective detergents according to the manufacturer's specifications remain indispensable preventive measures.
Keywords: B. cereus intoxication, coffee makers, potential risks, foodborne disease outbreaks, prevention
Introduction
Hot drinks vending machines (as defined by DIN), or fully automatic coffee makers, dispense a variety of coffee specialties and mixed milk or cocoa drinks. While microbiological problems are not commonly associated with black coffee, they are more likely to occur during the preparation and dispensing of the beverage if milk, as a foodstuff susceptible to contamination, is added. Depending on the type of coffee maker, milk is available as refrigerated whole milk, UHT milk or milk powder (topping). Coffee makers are available in Bundeswehr dining halls as well as (privately run) recreational facilities, both in Germany and in theatre.
To be able to assess the potential risk of contamination with spore- and toxin-producing B. cereus, coffee makers in several such facilities were examined for the presence of B. cereus and its ability to produce toxins.
Material and Methods
Between October and November 2015, a total of 22 coffee makers were examined in 21 Bundeswehr dining halls and recreational facilities in the German states of Rhineland-Palatinate, North Rhine-Westphalia, Hesse and Saarland.
Samples were taken after the machines were cleaned, which, according to the manufacturer's specifications, must be done daily. Cleaning involves various steps depending on the manufacturer and model as well as various cleaning intervals, as the following example of a common model shows:
- daily cleaning (incl. emptying coffee grounds tray) of all systems that were used at least once (brewing and instant system);
- cleaning prior to use if the machine or one of the systems has not been used for more than one week;
- monthly cleaning of casing components and filter screen inside the water inlet (if applicable).
Daily cleaning involves emptying the coffee grounds and selecting a self-cleaning program ("clean and switch off" to clean the instant and brewing system and switch off the machine afterwards; "intermediate clean" if the machine is used again immediately after cleaning). To clean individual systems, the programs "clean brewing system" or "clean instant system" are available.
During the daily cleaning process of the brewing system the prompt "insert cleaning agent" appears on the display. The cleaning program starts automatically once the cleaning tablet recommended by the manufacturer (e.g. Coffee Cleaner Tabs) has been inserted into the slot. For daily cleaning of the instant system, the mixing components need to be removed and cleaned either with hot water and a cleaning agent suitable for dairy products or in the dishwasher. The components should finally be rinsed with clear water. The instant container should be cleaned in the same way every week.
Samples taken from coffee makers:
- 1 to 3 samples of the milk used in various hot drinks, depending on the range of milk-based drinks offered;
- 1 sample of the topping powder in the container(s) inside the coffee maker;
- 1 original packaging of the same batch (if available) as the topping currently used.
Samples of topping powder were taken from the coffee maker with a sterile spoon. The powder was then filled into a sterile container. To take a sample of milk, a sterile 100 ml screw-top glass bottle was put under the outlet nozzle and a hot beverage with a high milk content (e.g. café au lait) was selected. Only the milk content of the drink was collected in the sample container. The outlet nozzle was not disinfected prior to this procedure in order to recreate normal conditions.
 Figure1: Example of topping/cocoa powder mixing unit in a coffee maker
(white arrow: milk/cocoa powder mixing pipe, blue arrow: hot water pipe, red arrow: coffee pipe)
Figure1: Example of topping/cocoa powder mixing unit in a coffee maker
(white arrow: milk/cocoa powder mixing pipe, blue arrow: hot water pipe, red arrow: coffee pipe)
Figure 1 shows the functional elements of an open coffee maker that dispenses milk in the form of topping powder.
The samples were tested in the laboratory according to the institute's test instructions. These describe the procedure for detecting B. cereus in cultures using colony counts in accordance with the official collection of test methods pursuant to § 64 of the German Food and Feed Code (LFGB, L 00.00-33). The sample material was mixed with buffered peptone water at a ratio of 1:10 and homogenized. A serial dilution was then prepared. Of each dilution step, 0.1 ml was spread over the surface of Mannitol Egg Yolk Polymyxin Agar (MYP Agar), a medium developed by Mossel. The inoculated agar plates were then incubated at 30 ± 1 °C for 18–24 h. Following the incubation period, all agar plates were examined for potential B. cereus colonies and enumerated. The bacterial count was based on all presumptive colonies on the selective agar medium (MYP) (figure 2). For the count, all agar plates with consecutive dilution steps on which colonies had grown were tested. On MYP agar, presumptive B. cereus colonies appeared as rough and dry cultures with a diameter of 2–5 mm against a violet-red background, generally with a white precipitate. Typical colonies growing on MYP agar plates as described above and also showing haemolysis on sheep blood agar could thus be identified as B. cereus.
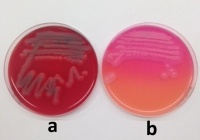 Fig. 2: Examples of B. cereus grown on sheep blood agar (a) and MYP agar according to Mossel (b)
Fig. 2: Examples of B. cereus grown on sheep blood agar (a) and MYP agar according to Mossel (b)
The Duopath® GLISA rapid test (Gold Labelled Immuno Sorbent Assay) was used to detect B. cereus enterotoxins. This test is an immunochromatographic screening and confirmatory test with gold-marked antibodies used to detect the diarrhoeal enterotoxins Hbl (haemolysin BL enterotoxin) and Nhe (non-haemolytic enterotoxin). Detection of cytotoxin K is not yet part of routine testing for B. cereus [17]; such a test was not performed as it would have yielded no additional information for our study.
To detect enterotoxins in the food sample, 10 g or 10 ml of the sample were homogenized with 90 ml CGY broth (casein hydrolyzate broth containing 1% glucose). Subsequently, 200 µl of the homogenized sample were mixed with 2 ml CGY broth in a 200 ml Erlenmeyer flask and incubated at 36 ± 1 °C for 18–24 h.
Depending on the toxin-producing ability of the isolates that had been isolated from the tested food sample and confirmed as B. cereus cultures, between one and three single colonies were transferred to 1 ml CGY broth, mixed and incubated at 36 ± 1 °C for 4 h. After cooling to room temperature, both samples were applied onto the Duopath® test device.
The test was conducted and evaluated according to the manufacturer’s instructions.
Results
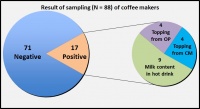 Fig. 3: Result of samples taken from coffee makers in 21 Bundeswehr facilities (OP = original packaging, CM = coffee maker)
Fig. 3: Result of samples taken from coffee makers in 21 Bundeswehr facilities (OP = original packaging, CM = coffee maker)
Samples were taken in 21 dining and recreational facilities in the German federal states of Rhineland-Palatinate, North Rhine-Westphalia, Hesse and Saarland. They included dining halls, various privately run smaller cafeterias and recreational facilities, facilities that serve food but do not prepare it on-site and one hospital kitchen. A total of 88 samples were examined for the presence of B. cereus, including 48 hot drinks and 23 topping powders from 22 coffee makers as well as 17 items of original packaging of the toppings used. Figure 3 shows the distribution of the samples contaminated with B. cereus.
B. cereus was detected in 11 (52%) of the catering facilities in which samples were taken. Bacteria could be isolated from 9 (19%) of 48 milk samples as used in various hot drinks and from 5 (22%) of 23 topping powders inside the machines as well as from 4 (24%) of 17 closed original packages. In one facility where food is served but not prepared on-site, B. cereus was detected in all three milk samples from hot drinks of the same coffee maker, at levels of 103–104 CFU/g. In one coffee maker in a junior ranks' club, the milk in two hot drinks as well as the original packaging showed contamination levels of 102 and 101 CFU/g, respectively. In another junior ranks' club, the milk content of one hot drink and the topping sample from the coffee maker were contaminated with B. cereus at concentrations of 103 and 101 CFU/g, respectively. In one privately run smaller cafeteria, B. cereus was isolated both from the milk content and the original packaging of the milk topping at a concentration of 102 CFU/g. In the other facilities, bacteria were detected only in one of the three samples.
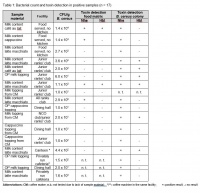
B. cereus cultures were identified in every one of the 17 positive samples. In 16 cases the Nhe and Hbl enterotoxin components were detectable in culture supernatants or in food samples. One sample could not be examined for the colony's ability to produce toxins as there was not enough material available. Both Nhe and Hbl enterotoxins were found in 6 (38%) of 16 B. cereus isolates tested, while the other 10 (62%) isolates apparently produced only the Nhe enterotoxin. Of the 14 milk samples from various hot drinks, 2 (14%) tested positive for both Nhe and Hbl enterotoxins. Another 2 (14%) milk samples tested positive for Nhe only. The other 10 (72%) samples did not contain either enterotoxin. Owing to the small amount of material available, three samples could not be tested for the Nhe and Hbl enterotoxins. Only one sample (latte macchiato milk content) showed evidence of both enterotoxins in both the sample itself and the colony, with B. cereus detected at a concentration of 1.1 x 103 CFU/g. Table 1 provides a detailed overview of all samples contaminated with B. cereus.
Discussion
B. cereus
B. cereus is one of seven species in the B. cereus group that are phylogenetically indistinguishable. They are generally categorized according to the diseases they cause. The B. cereus group (B. cereus sensu lato) comprises the following species:
- B. cereus sensu stricto: facultative pathogen that causes foodborne as well as local and systemic diseases;
- B. anthracis: obligatory pathogen that causes anthrax;
- B. thuringiensis: facultative pathogen used as biological pesticide because of its ability to produce insecticidal toxins; human pathogen;
- B. mycoides, B. pseudomycoides, B. weihenstephanensis: psychrotolerant pathogen that causes food spoilage;
- B. cytotoxicus: thermotolerant pathogen that causes foodborne diseases.
B. cereus is a Gram-positive, rod-shaped, motile, facultative anaerobe which, as an endospore-forming organism, is very resilient. Its robust spores are resistant to dry heat, radiation, extreme temperatures and fluctuating pH values. As the vegetative cells are able to produce biofilms, they are also resistant to various cleaning and disinfection agents [11, 16]. Biofilm formation is not only a typical feature of the vegetative cells of B. cereus; the spores also have an enormous adhesive ability. They are even able to adhere to the surface of stainless steel over longer periods [8].
Incidence
B. cereus is an ubiquitous pathogen commonly found in the environment, including in dust and soil. Low-level contamination with B. cereus or its spores is thus not uncommon in many plant-based or animal-based foods. According to the European Food Safety Authority (EFSA), contamination levels below 100 CFU/g are not unusual. Introduction of B. cereus at such low concentrations into other foods via contaminated source material, such as spices or milk powder, does not pose a health risk as defined by consumer health protection regulations [8].
Pathogenicity
An important aspect of pathogenicity is the ability of B. cereus to produce toxins, which cause extraintestinal and gastrointestinal symptoms. Toxin production plays a significant role in the context of foodborne infections and intoxications [3, 13, 25]. Two kinds of toxins are generally distinguished: diarrhoeal toxins (enterotoxin) and emetic toxins (cereluide).
The enterotoxin family consists of non-haemolytic enterotoxin (Nhe), haemolysin BL (Hbl) and cytotoxin K. These three toxins are genetically determined and are produced and released in the intestine after ingestion of food contaminated with B. cereus. Nhe is an enterotoxin complex of three protein components of different sizes whose genes (nheA, nheB, nheC) constitute an operon. These three components must be present in a specific ratio to achieve maximum cytotoxicity, which induces cell lysis through pore formation [10, 15]. More than 90% of B. cereus strains have the ability to produce Nhe enterotoxin [12, 24]. Hbl enterotoxin also consists of three protein components. Their combined action is required to induce cytotoxic activity. These genes (hblA, hblC, hblD) are also clustered into a common operon [19]. Like the Nhe enterotoxin, the Hbl toxin has a cytotoxic effect through forming pores, which also triggers cell lysis. Approximately 40–60% of B. cereus strains are able to produce the Hbl enterotoxin [18]. Cytotoxin K is a single-component toxin and consists of a protein that seems to have a cytotoxic, necrotizing and haemolytic effect. To date, the CytK1 and CytK2 forms have been identified. Production of this toxin is evident in 40–60% of B. cereus strains [21]. Pore formation by the above enterotoxins leads to a loss of sodium and chloride ions, resulting in electrolyte imbalance and a considerable loss of fluid. Symptoms usually occur 8–16 hours after ingestion of the contaminated food and include nausea, abdominal cramps and watery diarrhea [20, 23]. Combined action of all three components is necessary to achieve maximum biological activity of the enterotoxins. This means that detection of all three components of the enterotoxin complex is required in order to reliably assess the enteropathogenic potential of a B. cereus strain [16].
Strains producing the emetic toxin (cereluide) are intoxicating agents as opposed to enterotoxin-forming pathogens. Intoxication occurs when food containing sufficient amounts of preformed toxins is consumed. The fact that toxins are resistant to heat, acid and proteolysis and pH-stable has always been a major challenge for the food industry, particularly when it comes to food processing [13]. While vegetative bacteria can be inactivated or even eliminated through heating processes, sterile filtration or bactofugation, this has proven ineffective for the toxin [5]. The incubation period after ingestion of a toxic dose of 8–10 µg/kg body weight is relatively short (1–5 hours). Once the toxin is ingested, it binds to 5-HT3 serotonin receptors in the small intestine, stimulating the afferent vagus nerves, which causes vomiting [1, 23].
In most cases, enterotoxins only cause diarrhoea once they are produced in the intestine, which means that this kind of intoxication is caused if food contaminated with a sufficient number of B. cereus spores or vegetative cells is ingested. In rare cases, preformed enterotoxins are present in food but will normally not withstand passage through the stomach.
Intoxication
Most reported outbreaks are associated with the foodservice industry. This has been attributed to improper temperature control of cooked foods, such as failure to rapidly cool and refrigerate, inadequate cooking temperatures and foods being kept warm for too long at temperatures that are too low. Bundeswehr facilities are therefore required to keep meals hot at temperatures of at least 65 °C to prevent B. cereus spores from germinating [2]. It is undisputed that the pathogen causes the most problems in the food industry and particularly in the context of foodservice. The focus remains on foodborne intoxications associated with meals prepared in industrial kitchens and served in dining halls.
Prevalence
B. cereus is not on the list of notifiable pathogens under Section 7 of the German Infection Protection Act. It is thus for the most part only identified as the cause of foodborne disease in the event of an outbreak. The resulting limited availability of data means that there is most likely a high number of unreported cases of B. cereus intoxications. Even if the risk of B. cereus infection is considered to be low, instances of foodborne intoxication caused by this pathogen have increased dramatically over the last few years. In 2011, the EFSA reported a 122.2% increase in foodborne disease outbreaks caused by B. cereus toxins throughout the EU compared to the previous year [9].
Significance of B. cereus for the Bundeswehr
Outbreaks, particularly on operations, can have a considerable impact on the ability of a unit to fulfill their mission. Whereas bacterial infectious diseases/intoxications are often foodborne, norovirus, for example, is a common cause of gastrointestinal disease outbreaks through smear infection via toilets, taps and/or door handles [6]. Kleer et al. conducted an epidemiological study of frequently occurring gastrointestinal symptoms in the Bundeswehr between 1994 and 1997 and concluded that 43% of individual cases and 52% of outbreaks were caused by B. cereus, most of them transmitted in dining facilities [13]. This correlates with findings by Ernst, who examined outbreaks in 92 Bundeswehr dining facilities in North Rhine-Westphalia, Rhineland-Palatinate, Hesse and Saarland in 1999 and 2000. He detected B. cereus in 13.7% of the 4412 surface samples taken in areas where food was prepared and served [6]. Coffee makers were not included in these studies, although they pose a high risk of contamination/infection on operations owing to their widespread use in dining facilities.
Evaluation of the test results
In our study we focused on the health risks of diseases associated with B. cereus toxins which, in a foodservice context, can quickly develop into an outbreak that affects numerous patients.
The findings clearly show that ready-to-drink hot drinks and various toppings from coffee makers as well as the original packaging of topping powders in dining and recreational facilities are contaminated with B. cereus. Therefore, they pose a risk to the health of consumers that should not be underestimated. Coffee makers should thus be included in all epidemiological testing to investigate outbreaks. A microbiological analysis of coffee makers connected to the water supply network which was part of a study by Maczewski et al. (2015) confirms this. They too detected B. cereus and numerous Gram-negative pathogens, some of them hazardous to health [16].
Our tests also showed that both the original packaging and the topping samples taken from coffee makers contained B. cereus at low concentrations of 101–102 DFU/g. In January 2016, the German Society for Hygiene and Microbiology set the threshold for critical levels of contamination in instant products at 103 CFU/g food [4]. In the milk content of the different hot beverages, the concentrations of bacteria detected ranged between 102 and 106 CFU/g, which is considerably higher than the critical value of 103 CFU/g. According to EFSA reports, foodborne disease caused by B. cereus is associated with concentrations of vegetative cells or spores of 105–108 CFU/g food. In some cases, however, outbreaks have been observed as a result of concentrations as low as 103–104 CFU/g food [8]. The high bacterial count found in ready-to-drink coffee specialties indicates that parts of the machine are contaminated. This suggests that, for instance, hose systems are contaminated with bacteria as a result of biofilm formation.
What is also important to note is that of the ten coffee makers sampled in dining halls, only three were contaminated with B. cereus. None of the ready-to-drink hot beverages were contaminated. B. cereus was isolated at a low concentration of 101 CFU/g from only two items of original packaging of the topping powder used and from one topping sample taken from inside a coffee maker. One reason could be that dining halls usually serve a considerably higher number of customers every day than recreational facilities. Particularly at lunchtime, the coffee makers are used quite extensively, which means that all parts of the machine, especially the hoses, are regularly flushed with hot water. The operators of the coffee makers must further clean the machines in accordance with the intervals and instructions prescribed by the manufacturer. The test results could indicate that kitchen staff in dining halls comply more closely with the manufacturer's instructions.
B. cereus is generally difficult to eliminate as the spores are very resilient. Moreover, there is only a limited choice of cleaning and disinfection measures available for coffee makers as they need to be compatible with the material and technical design of the machine. The cleaning tablets supplied by the manufacturer must be used regularly. The composition of these cleaning tablets varies slightly depending on the manufacturer and the type of use (private household/foodservice industry). Most cleaning tablets used for coffee makers in foodservice facilities contain active oxygen in the form of sodium carbonate peroxyhydrate as a disinfecting component and potassium carbonate as a cleaning agent.
Peroxides such as hydrogen peroxide and peracetic acid are highly potent disinfection agents that demonstrate broad-spectrum activity, in particular against spores [23, 7]. Thanks to their residue-free decomposition, these substances are considered neither harmful to health nor the environment. Material compatibility of peracetic acid is limited but considerably improved by adding corrosion inhibitors. Although inactivated to some degree by protein and cold temperatures, that can countered with higher concentrations and longer exposure times. However, higher concentrations of peracetic acid must be used with caution because of the associated risk of irritation of the mucous membranes. Alkalization, however, may lessen this effect. The most well-known commercial preparations are Wofasteril® and PES 15/23.
Another option would be to shorten the cleaning intervals. Preparations with a high concentration of hypochlorite (pH value >8) are particularly suitable for piping systems as they achieve a considerable reduction or elimination of spores. Thermal disinfection is another option for treating machine components. Thermal disinfection at temperatures exceeding + 105 °C for at least three minutes leads to at least a 5-log reduction and can even kill all spores [8].
Using various preventive strategies to combat B. cereus as a cause of foodborne disease remains indispensable. This does not only apply to industrial food processing and the production of ready-to-eat meals for dining facilities. Cleaning and disinfecting the equipment, materials and articles that come into contact with food is also of utmost importance.
Conclusion
Gastrointestinal infections caused by B. cereus continue to be a risk in dining facilities which should not be underestimated. Combatting B. cereus continues to prove difficult as symptoms are usually mild, the pathogen is ubiquitous in the environment – and thus to a small extent also in food –, its ability to form spores makes it very resilient and its ability to produce toxins (high producers) varies. Because it is not notifiable and therefore many cases likely go unreported, there is, however, a danger of losing sight of B. cereus as a cause of foodborne diseases.
The data collected as part of this study should provide sufficient motivation to remain conscious of potential health risks due to poor hygiene, in particular in dining facilities. Ignoring the equipment-specific cleaning and disinfection intervals and procedures for coffee makers bears a risk that should not be underestimated. On the one hand, this results from adding milk topping, which is per se susceptible to B. cereus and, as this study has shown, may already be contaminated prior to use in the coffee maker. On the other hand, powder residue remaining in the piping system may lead to further bacterial growth or the formation of spores, which can ultimately lead to contamination of the ready-to-drink hot beverage at levels that are hazardous to the health of consumers.
To ensure effective consumer health protection, coffee makers used in dining facilities must be included in regular hygiene checks. Samples may be taken as described in this study. In addition, samples should also be taken from the machine's functional elements (hoses or any parts that are difficult to clean, such as valves or nozzles). Hygiene standards on operations must be the same as in Germany. Therefore, the guidelines for hygiene status controls in theatre should be supplemented accordingly for the veterinary medicine field laboratories. The recommendation to include coffee makers in hygiene status controls also applies to civilian consumer protection.
Last but not least, food hygiene experts should be consulted whenever coffee makers are procured to assess the cleaning and disinfection procedures prescribed by the manufacturer and, in particular, approved disinfection agents.
Key statements
- Because of its ability to produce toxins, B. cereus must not be underestimated as a cause of foodborne infections.
- Coffee makers and topping powders bear a health risk in the form of B. cereus food intoxications.
- Particularly in recreational facilities such as privately run smaller cafeterias and clubs, all parts of a coffee maker that come into contact with topping powder should be cleaned and disinfected on a regular basis.
- The cleaning regimen specified by the manufacturer must be followed and should be supplemented as required.
- Coffee makers as well as the ready-to-drink hot beverages served and the topping powders used should be included in regular hygiene checks and risk analyses with regard to foodborne outbreaks.
Literature
- Agata N, Ohta M, Mori M, Isobe M: A novel dodecadepsipeptide, cereluide, is an emetic toxin of B. cereus. FEMS Microbiol. Lett. 1995; 129: 17 – 20.
- Bundesinstitut für Risikobewertung (BfR) "Warmhaltetemperatur von Speisen sollte über 65 °C betragen", Comment No. 008/2008 of BfR dated 14 January 2008.
- Bennet SD, Walsh KA, Gould HL: Foodborne Disease Outbreaks caused by B. cereus, Clostridium perfringens, and Staphylococcus aureus-United States, 1998-2008. Clin Infect Dis. 2013; 57(3): 425 – 433.
- Deutsche Gesellschaft für Hygiene und Mikrobiologie (DGHM). Veröffentlichte mikrobiologische Richt-und Warnwerte zur Beurteilung von Lebensmitteln. (as of 22 January 2016).
- Ehling-Schulz M, Fricker M, Scherer S: Identification of emetic toxin producing B. cereus strains by a novel molecular assay. FEMS Microbiol. Lett. 2004; 232: 189 – 195.
- Ernst C: Optimierung von Desinfektionsverfahren in Verpflegungs- und Betreuungseinrichtungen der Bundeswehr im Hinblick auf die Bacillus cereus-Belastung von Oberflächen und Lebensmitteln: Dissertation in Veterinärmedizin an der Freien Universität Berlin, 2003; http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001034
- Ernst C, Schulenburg J, Jakob P et al.: Efficacy of amphoteric surfactant- and peracetic acid-based disinfectants on spores of bacillus cereus in vitro and on food premises of the German armed forces. J Food Prot 2006; 69(7): 1605 -1610.
- European Food Safety Authority (EFSA): Opinion of the Scientific Panel on biological hazards (BIOHAZ) on B. cereus and other Bacillus spp. in foodstuffs. The EFSA Journal 2005; 175: 1 - 48.
- European Food Safety Authority (EFSA) and European Center of Disease Control (ECDC): The European Union Summary report on trends and sources of zoonoses, zoonozic agents and food-borne outbreaks in 2011. The EFSA Journal 2013;11(4): 3129 [250 pp].
- Fagerlund A, Lindbäck T, Storset AK, Granum PE, Hardy SP: B. cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 2008; 154 : 693 – 704.
- Hayrapetyan, H., Muller, L., Tempelaars, M., Abee, T., Groot., N., M., Comparative analysis of biofilm formation by B. cereus reference strains an undomesticated food isolates and the effect of free iron. International Journal of Food Microbiology 200 (2015), 72-79
- In`t Veld PH, Ritmeester WS, Delfgou-Van Asch EH, Dufrenne JB, Wernars K, Smit E, Van Leusden FM: Detection of genes encoding for enterotoxins and determination of the production of enterotoxins by HBL blood plates and immunoassays of psychotrophic strains of B. cereus isolated from pasteurized milk. Int J Food Microbiol.2001; 64 (1-2): 63 – 70.
- Kleer J, Bartholomä A, Levetzow R et al.: Bakterielle Lebensmittel-Infektionen und Intoxikationen in Einrichtungen zur Gemeinschaftsverpflegung 1985 bis 2000. Arch Lebensmittelhygiene 2001 (52): 76-79.
- Kreienbrink G, Pöllein W, Fender T, Emmler J, Schotte U, Binder A: Lebensmittelbedingte Gruppenerkrankug in der Bundeswehr unter besonderer Berücksichtigung von Noroviren. Wehrmedizinische Monatsschrift 2012; 10: 240 – 246.
- Lindbäck T, Fagerlund A, RØdland M S, Granum PE: Characterization of the B. cereus Nhe enterotoxin. Microbiology 2004; 150(12): 3959 – 3967.
- Maczewski S, Graetz A, Winterfeld I, Jakob C, Mattner F: Stellen an das Trinkwassernetz angeschlossene Kaffeeautomaten ein Risiko für Patienten dar? HygMed 2015; 40 (9): 361 - 364
- Messelhäuser U, Ehling-Schulz M: Pathogene Mikroorganismen, B. cereus-Vorkommen, Nachweis und Präventionsstrategien. Hamburg: Behr`s Verlag; 2. Auflage 2014.
- Moravek KM, Dietrich R, Buerk C et al.: Determination of the toxic potential of B. cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 2006; 257: 293 – 298.
- Movarek KM.: Nachweis und Expression und Bedeutung der von B. cereus produzierten Enterotoxin-Komplexe. Dissertation aus dem Institut für Hygiene und Technologie der Lebensmittel tierischen Ursprungs (Lehrstuhl: Prof. Dr. E. Märtlbauer) der Tierärztlichen Fakultät der Universität München, München 2006; https://edoc.uni-muenchen.de/5714/ (accessed: 15 September 2016).
- Reis ALS, Montanhini, MTM., Bittencourt JVM, Destro TM, Bersot LS: Gene detection and toxin production evaluation of hemolysin BL of B. cereus isolated from milk and dairy products marketed in Brazil. Braz. J. Microbiol. 2013) 44(4): 1195 – 1198.
- Stenfors Arnesen LP, Fagerlund A, Granum PE: From soil to gut: B. cereus and its food poisoning toxins. FEMS Microbiol. 2008; 32(4): 579 – 606.
- Sudhaus N, Pina-Pérez MC, Martínez A, Klein G: Inactivation Kinetics of Spores of Bacillus cereus Strains Treated by a Peracetic Acid–Based Disinfectant at Different Concentrations and Temperatures. Foodborne Pathogens and Disease 2012; 9(5): 442 - 452.
- Ueda S, Yamaguchi M, Iwase M, Kuwabara Y: Detection of Emetic B. cereus by Real-Time PCR in Foods, Biocontrol Science, 2013; 18(4): 227 – 232.
- Wijnands LM, Dufrenne JB, Rombouts FM, In`t Veld PH, Van Leusden FM: Prevalence of potentially pathogenic B. cereus in food commodities in the Netherlands. J Food Prot. 2006; 69 (11): 2587 – 2594.
- Zhang Z, Feng L, Xu H, Liu C, Shah NP, Wei H: Detection of viable enterotoxin-producing B. cereus and analysis of toxigenicity from ready-to-eat foods and infant formula milk powder by multiplex PCR. Journal of Dairy Science 2016; 99(2): Article in press; https://www.reserachgate.net/publication/287388321 (Accessed: 15 September 2016)
Images courtesy of:
Figures 1 and 2: B. Bovermann, Mainz
Citation format:
Bovermann B: Bacillus cereus contamination in coffee makers at several Bundeswehr dining and recreational facilities Wehrmedizinische Monatsschrift 2017; 61(2/3): 42 – 49.
Written by:
Oberstabsveterinär Birte Bovermann
Central Institute of the Bundeswehr Medical Service in Koblenz,Mainz branch
Laboratory Department II, Veterinary Medicine
Generaloberst Beck Str. 1f, 55129 Mainz
Email: BirteBovermann@Bundeswehr.org
Date: 07/06/2017
Source: Wehrmedizinische Monatsschrift 2017/2-3









