
Article: H. SUDECK (GERMANY)
HAEMORRHAGIC FEVER IN THE FIELD
Crimean-Congo haemorrhagic fever – a significant risk in the field?
After dengue fever, Crimean-Congo haemorrhagic fever is of tick-borne arbovirus-induced disease worldwide. One of the unusual features of this essentially zoonotic virus is the way that it is transmitted. Infection in humans is not only the result of tick bites, but the virus can also be transmitted from human to human. This article looks at the clinical aspects – diagnostics, treatment and prevention and outlines the strategies developed by the Tropical Medicine Unit of the German Armed Foces Military (Bundeswehr) Hospital Hamburg to battle this biological threat.
Introduction
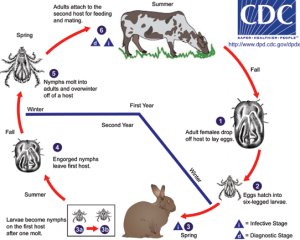 Life-cycle of Hyalomma ticks.
Life-cycle of Hyalomma ticks.
Crimean-Congo haemorrhagic fever (CCHF) is a typical tick-borne arbovirus-induced disease (‘arbovirus’ is an acronym for ARthropod-BOrne virus; it is transmitted by mosquitoes and ticks). After dengue fever, CCHF is the most common form of this disorder worldwide. The Crimean-Congo virus (CCV) is also one of the first described and most widely disseminated haemorrhagic fever viruses (HFVs). In recent months, there have been escalating numbers of reports of cases of infection in the Bangladesh/Pakistan region and the border areas between Afghanistan and India. The death of a member of the US personnel serving with the ISAF in 2009 clearly demonstrates the potential risk here, while it has long been known that the disease is endemic in the region in which the KFOR operates. fOne of the unusual features of this essentially zoonotic virus is the way that it is transmitted. Infection in humans is not only the result of tick bites, but the virus can also be transmitted from human to human following contact, particularly with blood or secretions. The natural reservoir
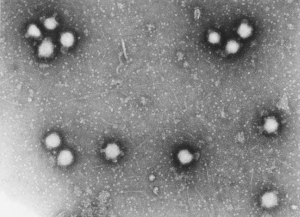 Electron microscopy of CCHV.
Electron microscopy of CCHV.
It is quite possible that the earliest account of infection with Crimean-Congo virus is that found in the ‘Kitab al-Hawi’ (a medical textbook) composed by the Persian clinician Abu Bakr al-Razia, also known as Rhazes (c. 854 – 932). He describes three cases in which the symptoms exhibit a remarkable similarity with those of Crimean-Congo haemorrhagic fever. Subsequently, a follower of Avicenna, the 12th century Persian royal physician Husayn Gorgani, also described a case in Tajikistan in which the cardinal symptoms were blood in the urine, vomiting and blood-stained sputum. He postulated that the infection had been transmitted by an arthropod and proposed that “parasites in black birds” were the source. Similar disease syndromes have been recorded for centuries in the popular culture of the Uzbeks.
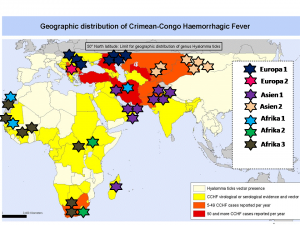 Distribution of CCV and its subtypes in the world.
Distribution of CCV and its subtypes in the world.
It was in the Belgian Congo in 1956 that the first identified case of infection with Crimean-Congo virus emerged in more recent times. On 6 March 1956, a 13-year-old boy with fever, headache, vomiting, generalised joint pain and photophobia reported to the practice of a Belgian colonial physician in Stanleyville. Blood samples were taken from the boy and administered by the intracerebral and intraperitoneal routes in a mouse. This test animal and all subsequent test mice exposed to samples became ill or died and the physician also developed a disorder characterised by fever, nausea and vomiting; blood samples were also taken from the physician and used to inoculate test mice. As no freezer was available, the isolate resulting after passage through a bacteria-proof filter was sequestered in mice and transported in this form to the research institute in Entebbe (now the Uganda Virus Research Institute) – two laboratory personnel there then also fell ill with a disorder presenting with similar symptoms. But it was only in 1967 that the first relevant publication appeared under the title Congo virus, a previously unknown virus from Africa. At the same time, the Russian virologist Mikhail Chumakov was investigating a virus that had been isolated in the Crimea and which he was using to induce therapeutic fever in psychiatric patients. The Soviet authorities had established the viral aetiology of the related disorder by means of passage through human ‘volunteers’ and inoculation of mice. The number of fatalities resulting from this experiment has not been reported. In 1944, some 200 Russian soldiers stationed in the Crimea had developed febrile, haemorrhagic symptoms while helping local peasants suffering from the consequences of the war. Chumakov was able to demonstrate the presence of the corresponding virus in the Hyalomma tick and the Soviets thus insisted that the newly identified pathogen be called Crimean-Congo haemorrhagic fever virus rather than Congo-Crimean haemorrhagic fever virus. The abbreviation CCHF serves, of course, for both versions. It is thus an historical fact that it was the politics of the Cold War that in this case determined virus taxonomy!
Virology
Crimean-Congo virus is a member of the Bunyaviridae family. This includes the genuses Hantavirus, Phlebovirus and Nairovirus. CCV is a Nairovirus, all of which are transmitted by ticks. It is passed on within the vector by means of transoverial and transstadial transmission. The life cycle (Figure 1) and passage from tick to vertebrates can involve not only wild animals but also livestock and pets, domesticated birds, such as the ostrich, and ruminants, such as the camel. Within its coat, the virion has three RNA sections that are known as the Small (S), Middle (M) and Large (L) segments and it has a diameter of 90 – 100 nanometres (Figure 2). Under an electron microscope, it can be readily differentiated from other members of the Nairovirus genus. Thanks to molecular-genetic sequencing of the S segment, various genotypes have been identified that occur in different geographical regions; hence there are the subtypes Asia 1 and 2, Europe 1 and 2, Africa 1, 2 and 3, and eight clades with some 70 genetically diverse genotypes and eight clades [IC1]that also appear to exhibit differing levels of virulence and viral ecologies (Table 1). It is probable that both reassortment and recombination are responsible for this diversity.
Clade | Occurrence |
1 | Europe, Southeastern Russia, Turkey |
2 | Greece (in the Rhipicephalus tick only) |
3 | Central Asia: Tajikistan, Uzbekistan, Kazakhstan, China |
4 | Iran, Madagascar, Pakistan |
5 | Iran, Senegal, Mauretania |
6 | Senegal, Mauretania, South Africa |
7 | Nigeria, Central African Republic |
8 | Uganda |
Table 1: Clades and geographical distribution
Transmission, ecology and occurrence
For a general overview of the distribution of the various subtypes worldwide and in the regions in which the Bundeswehr is currently operating, see Figure 3.
 Hyalomma tick.
Hyalomma tick.
It has been demonstrated that 31 tick species of the Ixodidae family act as vectors. Hyalomma (Figure 4) is the preferred genus, but Rhipicephalus, Boophilus, Dermacentor and Ixodes can also be infected. While it is the tick nymphs that tend to transmit the virus to smaller mammals, such as rabbits, hares and hedgehogs, the adult ticks can also infect larger (domestic) animals. Animals bitten by ticks do not usually fall ill. The disease can also be transmitted to humans when the vector is crushed against the skin of its host, for example, during sheep shearing. Common modes of infection transmission are the butchering of animals and contact with meat. There is a high risk of nosocomial and intrafamilial transmission (particularly if the infected person is haemorrhaging) and this is a serious problem for medical personnel. The possibility of aerogenic transmission has been postulated but this has not been confirmed. A case of diaplacentary transmission has been reported by the Turkish team led by O. Ergonul, one of the leading researchers in this field in Southern Europe.
Since the disease was first described in 1967, there have been some 140 reported outbreaks of CCHF involving more than 5000 individuals in 52 different countries. Of particular interest are the outbreaks that have occurred in NATO member country Turkey and the significant seasonal increase in the number of cases in the April to July period, particularly in Kosovo in the years 2008, 2009 and 2010. Cases of nosocomial infection of physicians and nursing personnel have occurred more frequently in the last years, particularly in association with outbreaks in Mauretania, Turkey, Bulgaria, India and Pakistan, and there have been reports of fatal outcomes. A review of ProMed mails of recent years shows that there has been a significant increase in reports of cases from Pakistan, Afghanistan and India, while the first report of the presence of CCV in Hyalomma ticks in Spain dates to 2010. Following numerous reported cases of suspected infection in Southern Sudan, a case of nosocomial transmission of the disease from an infected butcher was confirmed by means of molecular genetic analysis in 2010. In 2009, a US serviceman based in Southern Afghanistan who developed the relevant symptoms after being bitten by a tick died after evacuation to the hospital in Landstuhl; however, the screening of his comrades showed no evidence of transmission to them.
Clinical aspects
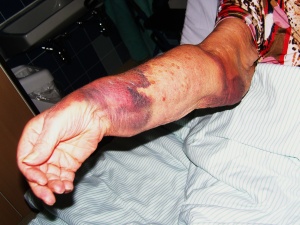 Typical findings in patients with hemorrhage.
Typical findings in patients with hemorrhage.
The clinical signs and symptoms of CCHF are similar to those of other viral haemorrhagic fever infections, such as Ebola, Marburg and Lassa. Its course is determined by the infective dose (MID: 1 – 10 viruses), the mode of acquisition and undoubtedly by the type of the virus. After an incubation period of approximately 3 – 7 days (the maximum period documented is 13 days), the patient experiences headache, non-relapsing fever, myalgia and dizziness in the prehaemorrhagic phase. There may also be diarrhoea, nausea and vomiting, while cutaneous hyperaemia, exanthema and conjunctivitis have also been described. About three days later, the disorder either spontaneously and unspectacularly remits or the haemorrhagic phase develops; the onset is abrupt and involves haemorrhaging from all organs. There have been cases in which this internal haemorrhaging has not been associated with the development of external stigmata. Two characteristic forms the disease can take are illustrated in Figures 5 and 6. Assuming the patient survives this phase, the convalescence phase commences some 10 – 20 days after onset and in this period the patient presents with generalised symptoms. From the 2008/2009 outbreak in Kosovo, it it has become retrospetively apparent that there can be significantly prolonged viraemia up to day 36 after infection. CCV is comparable in this respect with other HFVs, such as Lassa and Marburg. The Turkish research team under Ergonul have attempted to establish a method of prognosis using a few standard laboratory parameters.
- Platelets < 20,000/mm
- ALT > 900 U/ml or AST > 700 U/l
- aPTT > 60 sec. or fibrinogen < 110 mg/dl
indicate an unfavourable prognosis. Some authors include gastrointestinal haemorrhaging, somnolence and splenomegaly and patients who succumb to CCHF have significantly elevated levels of IL-6 and TNF-α in comparison with those who survive. The mortality rate differs from region to region and the rate usually quoted is in the region 20 – 50% (WHO: 30%). Death generally occurs in the first or second week of illness.
Pathogenesis
The pathogenesis of CCHF is often compared with that of Ebola and for this reason CCHF has been called “the Asian Ebola virus”. In common with all other haemorrhagic fever viruses, CCV affects the body on two levels; direct virus-induced cytopathic damage to the cells of organs with endothelial deterioration are accompanied by activation of the immune system and release of cytokines, chemokines, NO and other mediators that results in programmed cell death, stimulation of the coagulation cascade with development of DIC and multiorgan failure. Please see the relevant literature for more details.
Diagnosis and differential diagnosis
 Camp of ISAF troops with proper prevention measures.
Camp of ISAF troops with proper prevention measures.
Considerable care and thought are necessary in order to establish the anamnesis of a patient who may well subsequently be unable to provide information and the possibility of their exposure. As well as contact with ticks, it is advisable to ask the patient whether they have had contact with infected persons or animals and what outdoor activities they are involved in. In the case of service personnel, it is important to establish whether they have been on bivouac (Figure 7) or out on patrol or whether they have had contact with locals or animals. Even in desert regions, domestic sheep and goat herds can harbour ticks. (Figure 8), providing for the possibility of exposure. The appropriate biosafety measures must be in place when conducting laboratory tests to establish a diagnosis. One routine method employed is the detection of IgM and IgG antibodies by means of ELISA; the result on day 6 – 7 will be positive. IgM antibodies persist for 4 months while IgG antibodies are detectable for up to 5 years after infection – patients who rapidly succumb to the disease exhibit no detectable immune response. In order to prevent nosocomial spread of the disorder, it is essential to establish the diagnosis as rapidly as possible and in all appropriately equipped medical centres this can now be achieved within 8 hours with the help of reverse transcription PCR. The method was initially developed by personnel at the Bernhard Nocht Institute for Tropical Medicine in Hamburg, with which we collaborate. Another possibility is to isolate and incubate the virus, but this must be conducted under BSL-4 conditions and takes 2 – 5 days.
In differential diagnosis, it is important to distinguish CCHF from other viral haemorrhagic fevers that are endemic in the region in question; other possible bacterial pathogens include, in particular, Rickettsia (murine typhus and spotted fever and infections caused by Orientia tsutsugamushi in Asia), leptospirae, Borrelia recurrentis, meningococci and blood parasitoses, such as malaria and acute trypanosomiasis.
Treatment
When treating the corresponding patients it is essential, in both the civilian and military spheres and irrespective of the complicating factors obtaining in the field, to apply the principles of barrier nursing – self-protection must be given priority. In addition to aggressive supportive therapy of haemorrhaging and multiorgan failure, the drug of choice in use today is ribavarin, a purine nucleoside analogue first synthesized in 1972. As a polymerase inhibitor, it interferes with viral RNA and DNA replication in vitro, however, its in vivo efficacy is still the subject of dispute and this aspect may also be determined by the point in time at which it is administered – the earlier the better. There is an internationally recognised dosage schedule that is recommended by the WHO for administration by the oral route and the more expensive intravenous route. Ribavarin is available in the field hospitals in the relevant theatres of operations. The therapeutic efficacy of other strategies employed, such as the use of immune serums or subunit-specific monoclonal antibodies has not been confirmed in controlled trials while confirmation of these approaches is impractical because ethical considerations argue against the use of placebo controls over longer periods. In view of the possible alternative diagnoses, it is advisable to initiate additional antibiotic therapy or anti- Plasmodium falciparum treatment prior to diagnosis confirmation; the use of doxycycline (also in combination) can be recommended for this purpose.
Prevention
 Goats (and ticks) in the
desert.
Goats (and ticks) in the
desert.
Already well established in our military medical context are methods of mechanical and chemical vector prophylaxis that are employed in combination with continuing education of the deployed personnel. However, the level of awareness of other potential modes of infection is not yet sufficiently high among medical and combat personnel and nosocomial dissemination of the disease is a very real risk in the field. It is essential to reduce this risk by means of providing information on the disease, its signs and symptoms and continuous training, particularly of young medical officers prior to their deployment. With this in view, the Bundeswehr’s own Tropical Medicine Unit has designed a course to prepare the relevant medical personnel for deployment that covers aspects of infectiology and tropical medicine. The relatively high number of 200 cases reported from the Balkans in 2009/2010, the spread of the virus to the neighbouring NATO countries Turkey and Bulgaria, its confirmed presence in Spain and the death of the US soldier represent constant reminders and should spur us on to put in place the necessary precautionary measures. The exercise entitled Schneller Schutz (Rapid Protection) mounted by Hygienist Colonel Dr. Lüke with the collaboration of the 25th German KFOR contingent and our unit demonstrated in particularly graphic form how complex the real requirements for the treatment of such a patient or merely a suspected case actually are (Figure 9). At the same time, it has resulted in the implementation of a draft concept to cover this situation that will be used as a model for similarly structured exercises to be undertaken by the ISAF in Afghanistan. An appropriate step in the right direction is represented by the advanced barrier nursing course that will be offered by the Tropical Medicine Unit in Feldkirchen exercise hospital this coming autumn.
Conclusions
The areas in which the proliferating Crimean-Congo haemorrhagic fever is endemic overlap with several of the theatres of operations of the Bundeswehr. This arbovirus infection is thus a rare but serious threat, particularly to the personnel serving with ISAF and KFOR. Prophylactic measures and anticipatory planning of measures to care for infected military personnel or suspected cases, also among the civilian population, are required; the concepts that have already been drafted in this respect need to be more extensively tested and improved as necessary. The Tropical Medicine Unit of Hamburg Bundeswehr Hospital acts as a competence centre, providing both theoretical training of medical officers and a rapid reaction task force that represents the active element of special preventive medicine within the Joint Medical Service of the Bundeswehr.
Address of the author:
Bundeswehr Hospital Hamburg
Tropical Medicine Unit
Lesserstr. 180
22049 Hamburg / Germany
E-mail: [email protected]
Date: 09/27/2018
Source: Medical Corps International Forum 1/2013











