
Advertorial: Prytime Medical
Resuscitative Endovascular Balloon Occlusion of the Aorta: New Partial REBOA Device Allows 2+ hours of Safe Occlusion for Civilian and Military Utilization
1. A Brief History
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) emerged as a potential solution for controlling severe, uncontrollable bleeding during the Korean War[1]. However, technological limitations of the time prevented its widespread adoption. The concept was largely forgotten until the early 2000s when the challenges of treating traumatic injuries in the Iraq and Afghanistan wars reignited interest in REBOA. The U.S. military, facing the urgent need for effective hemorrhage control, invested heavily in research and development, leading to the creation of the first commercially available REBOA devices. These devices were designed for use in both combat and civilian critical care emergency settings.
2. From Complete to Partial Occlusion: Hemodynamic changes and organ perfusion
Initially, REBOA involved completely blocking blood flow in the aorta below a specific point, leading to essential hemodynamic changes. Complete REBOA increases aortic pressure above the occlusion site maintaining perfusion of the brain and the heart, while decreasing aortic pressure below the occlusion site results in distal ischemia to the kidneys and other organs. The latter limits the use of this occlusion strategy to 30 minutes in Zone 1 (supradiaphragmatic aorta for control of intra-abdominal bleeding) and 60 minutes in Zone 3 (infrarenal aorta for control of pelvic or junctional bleeding). To address this problem, techniques such as partial or intermittent REBOA, i.e. either maintaining a certain blood flow below the occlusion (partial REBOA) or intermittently opening the occlusion (intermittent REBOA), have been developed, with intermittent REBOA being associated with significant hemodynamic changes.
A significant breakthrough came with the development of the pREBOA-PRO™ catheter. The pREBOA-PRO™ catheter is the first and only FDA-cleared, and now CE Marked, REBOA catheter designed specifically for True Partial REBOA™ (Figures 1 and 2). Unlike its predecessors, this device allows for partial occlusion of the aorta, striking a balance between controlling bleeding and maintaining blood flow to essential organs. This innovation has broken the 30 minutes barrier in Zone 1 with reported safe occlusion times of longer than 2 hours, expanding the potential applications of REBOA and significantly increasing its effectiveness. With the ability to precisely titrate the level of occlusion and distal blood flow needed for the patient – like a dimmer switch™ – physicians can safely extend aortic occlusion time while reducing the risk of ischemic injury.
3. REBOA in the Modern Era
Today, REBOA is a critical tool in the arsenal of trauma surgeons and emergency physicians. The ability to precisely control aortic blood flow has transformed the management of severe hemorrhagic shock.
- Civilian Use: In civilian settings, REBOA has demonstrated its ability to stabilize critically injured patients, buying precious time for successful surgical planning and the recruitment of additional resources such as CT scanning and endovascular techniques. Moreover, it has been shown to reduce blood product requirements and improve patient outcomes, especially reduction in acute kidney injury.
- Military Applications: The battlefield has proven to be a demanding environment for REBOA. With prolonged evacuation times, the ability to maintain blood pressure and control bleeding with partial occlusion is invaluable. The Ukrainian military has successfully employed pREBOA-PRO™ in combat for prolonged evacuation transport of warfighters to safety, highlighting its potential as a lifesaving intervention in austere conditions. Ukrainians now report over 50+ uses in combat, the first case series of which has been submitted to the Journal for Special Operations Medicine (JSOM) for review (Akrish et al.[2]). The preliminary data from the usage of REBOA in Ukraine suggests that occlusion time is 4 times longer providing time for triage, treatment and transport. Importantly, those teams are now working with Prytime (Figure 3) to formalize an in-country combat data collection capability to aid in data collection funded by an MTEC grant. The US military is revising their REBOA Clinical Practice Guidelines to account for this new capability and the Bundeswehr has made a large investment in REBOA products and training.
- Pre-hospital transport: In Germany, REBOA has also been used in pre-hospital emergency medicine cases; however, it is not yet used regularly since many rapid response vehicles are not equipped with REBOA devices. Hilbert-Carius et al.[3] have reported their planning and training for use of REBOA for bleeding patients, as well as an adjunct for refractory out-of-hospital cardiac arrest in a German helicopter emergency medical service (HEMS). In that setting, the DRF-Luftrettung in Halle (Saale) Germany has incorporated REBOA in pre-hospital emergency medicine. Hilbert-Carius et al.[4] also reported two cases in which the procedure was used pre-hospital by a team from a rescue helicopter of the DRF Luftrettung (German air rescue). They concluded that “REBOA remains a treatment option for a few specially trained teams and a select group of patients, and some patients can certainly benefit significantly from it.” The ongoing RIBCAP-HEMS (REBOA In Bleeding and Cardiac Arrest in the Pre-hospital care by Helicopter Emergency Medical Service) project will collect further data on the potential REBOA integration into the pre-hospital care. In addition to the air rescue vehicle mentioned, REBOA is currently also available on the various Medical Intervention Cars (MIC) in Germany[5].
4. The Future of REBOA
Ongoing research and development continue to refine REBOA technology. Future advancements may include:
- Improved catheter designs for better placement and occlusion
- Integration of advanced imaging for real-time monitoring
- Development of algorithms to optimize REBOA use based on patient-specific factors
As our understanding of traumatic injury and hemorrhage control grows, REBOA is poised to play an even more critical role in saving lives.
Further information on ER-REBOA PLUS™ and pREBOA-PRO™ Partial REBOA Catheter can be found on https://prytimemedical.com and you can request further information on product distribution in Europe via [email protected].
Prytime Medical
12 Upper Balcones Rd, Suite 300
Boerne, TX 78006, USA
Tel: +1 210.340.0116
[email protected]
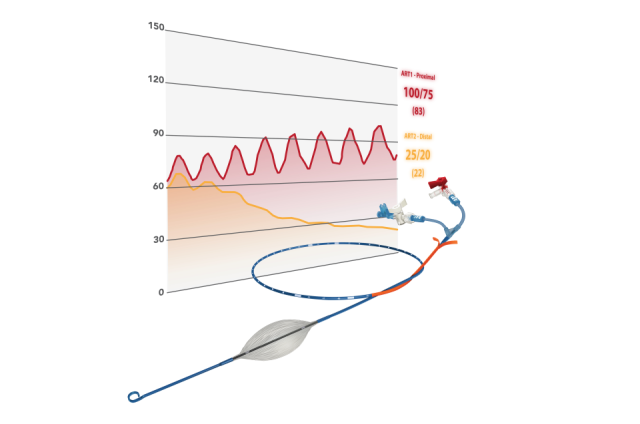
Figure 1: pREBOA-PRO™ Catheter and its components
The product consists of a semi-compliant balloon, an integrated pressure relief valve (safety valve), an atraumatic distal tip (P-tip™), a double lumen catheter shaft and a hub with extension lines that provide access to the respective lumen.
The catheter is designed with flow channels called "flow channels" that allow the inflation and deflation of the balloon to be precisely controlled and seamlessly managed.
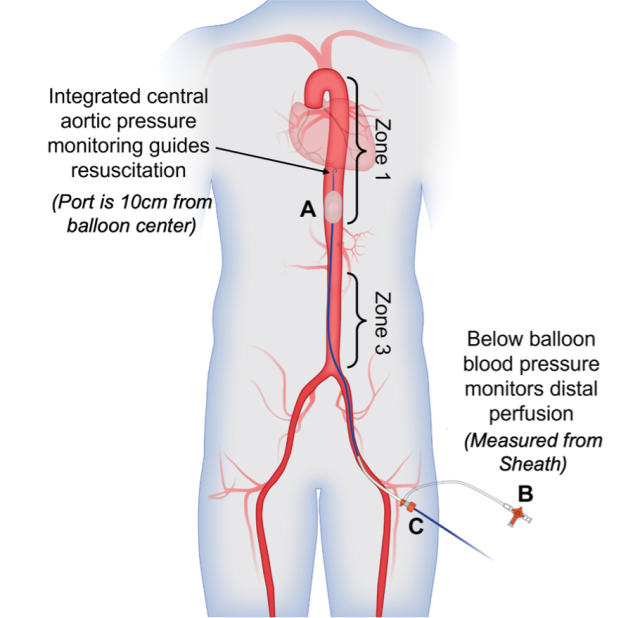
Figure 2: pREBOA-PRO Catheter deployed in zone 1.
A = Balloon with integrated aortic pressure measurement 10 cm above the balloon center
B = Balloon lumen stopcock, which contains an integrated safety valve that prevents the balloon from over-expanding.
C = Sheath where distal perfusion is monitored using balloon pressure.

Figure 3: REBOA training in Ukraine using the Prytime Medical Simulator (ARTI)
[1] Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954;36:65-8.
[2] Akrish, EO, et al, “Battlefield Endovascular Resuscitation in Ukraine: A Pragmatic Guideline for Partial Resuscitative Endovascular Balloon Occlusion of the Aorta” submitted to Journal of Special Operations Medicine.
[3] Hilbert-Carius P, McGreevy DT, Abu-Zidan FM, Hörer TM; the ABOTrauma Registry research group. Pre-hospital CPR and early REBOA in trauma patients - results from the ABOTrauma Registry. World J Emerg Surg. 2020 Mar 30;15(1):23. doi: 10.1186/s13017-020-00301-8. PMID: 32228640; PMCID: PMC7104487.
[4] Hilbert-Carius P, Hauer T, Josse F, et al. REBOA – Resuscitative Endovascular Balloon Occlusion of the Aorta. NOTARZT. 2020;36:33–45.
[5] Kaltschmidt N, Katzenschlager S, Kaltschmidt L, Leo A, Weilbacher F, Popp E. Materielle Ausstattung des Heidelberger Medical Intervention Car. Notfall + Rettungsmedizin. 2024.
Date: 10/17/2024











