
Article: Emma Loebler, Anton Chavdarov, Nigel Talboys, Erwin Dhondt, Andre Cap, Peter Tulkens
Ensuring the security of supply of blood and blood products
Overview
On 19 September 2024, FIPRA hosted a roundtable discussion on securing the supply of blood and blood products to ensure operational readiness for a range of civilian and military threats.
A wide range of stakeholders participated including the European Commission, NATO, the European Blood Alliance, national blood establishments and other stakeholders. The roundtable was conducted under Chatham House rules with sponsorship from Cerus and Terumo Blood and Cell Technologies (BCT).
The roundtable took place at a critical juncture: ahead of the confirmation of the European Unions (EU) new College of Commissioners and following the nomination of the bloc’s first ever Defence Commissioner, tasked with assessing the Union’s preparedness for a major conflict.
The discussion focused on three main aspects of blood supply in the context of conflict: governance and competencies in blood supply management; innovation and innovative products; and civilian and military cooperation.
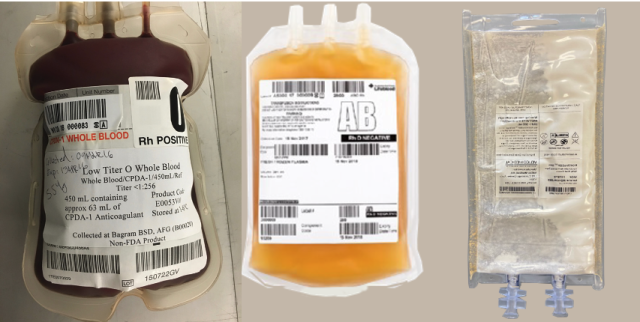
From left to right: Low Titer O Whole Blood (LTOWB) bag; Wet and dried blood plasma bags (source: Andrew P. Cap)
The problem
While often taken for granted and overlooked in higher-level planning, the supply of blood is essential for the functioning of national health systems and can be easily put under strain through cost constraints and a focus on minimising waste, as happened during the COVID-19 pandemic. The provision of robust blood supply logistics has also been proven to be one of the key determinants for improved survival rates in conflicts. The invasion of Ukraine by Russia has brought back large-scale warfare to the borders of Europe, putting a spotlight on the EU’s national and collective preparedness for any escalation.
The EU, NATO and national civil and military systems, along with other stakeholders, must work together to develop processes and systems that ensure resilience in blood supply in the event of a crisis, be it health, natural disaster or conflict related.
The roundtable started with a presentation on an issue that affects both civil and military settings, namely the lack of capacity for broad deployment of blood products to trauma patients in the emergency window, and the lack of stockpiles of relevant products to manage large casualty producing events, further complicated by a number of cyber and supply-chain vulnerabilities.
Participants discussed the lessons from the Russian invasion of Ukraine and the stress it had put on the supply of blood. While blood use in Ukraine had gone up 40% compared to pre-war levels, there was no shortage of donors, signifying that in times of crisis, the donor population becomes more active. Issues were, however, encountered in the state’s ability to process the donations due to a lack medical devices and low levels of automation.
Governance and Competence
On the landscape of regulation, participants emphasized the need for multi-stakeholder cooperation to ensure interoperability and the harmonisation of technical standards. Participants highlighted labelling and crisis care as two areas where standardisation could make a large difference in a cross-border situation.
At the regulatory level, participants highlighted the need for harmonisation between key instruments including the EU’s new Substances of Human Origin Regulation (SoHO), national laws, and the NATO Standardisation Agreements (STANAG). Speakers agreed that while the old EU Blood Directive had its challenges, the SoHO Regulation provided solutions to many of the problems related to blood supply. In particular, they pointed to Article 63 (drawing up of National SoHO emergency plans with cooperation with the military), Article 65 (addressing the need for self-sufficiency) and Recital 66 (stating the need for stockpiling of critical products), as crucial steps in the right direction. Attendees further underlined the importance of having a common base-line standard for blood products to be reverted to during emergencies and acknowledged the progress made under the SoHO Regulation and European Directorate for the Quality of Medicines & HealthCare (EDQM) Blood Guide to provide such a baseline.
However, participants noted several persisting challenges to successful cooperation including political will and funding, as well as limitations in existing governance. For example, while Ministries of Foreign Affairs often have strong links to the military, the Ministries of Health responsible for blood policy often don’t sit in the relevant conversations.
While there was agreement that cross-border cooperation will be key in a crisis, the building of surge capacity should happen at national level. Participants agreed that this is only possible if gaps in national capacity are understood. It was noted that the SoHO regulation requires member states to have emergency plans in place at national and blood centre level and that these were essential in case of a crisis. However, these plans were not originally designed with military intent and do not include a requirement for cross-border collaboration nor a minimum competency to produce certain blood products that may be needed in other regions in a crisis.
Attendees also highlighted best practice examples from around the world for stockpiling and blood centre cooperation, such as the US Blood Emergency Readiness Corps (BERC), and spoke of the benefits of similar systems being implemented in the EU to strengthen cross-border collaboration and response.
Finally, participants discussed the role that the Health Emergency Preparedness and Response Authority (HERA), a directorate-general of the European Commission, could play at the EU level given their current responsibility for civilian preparedness and the stockpiling of medical countermeasures in Europe.
The question was raised as to whether the “rescEU” stockpiles, as part of the EU Civil Protection Mechanism, would be used for the military depending on the crisis and participants discussed the importance of including blood in the list of essential medical countermeasures.
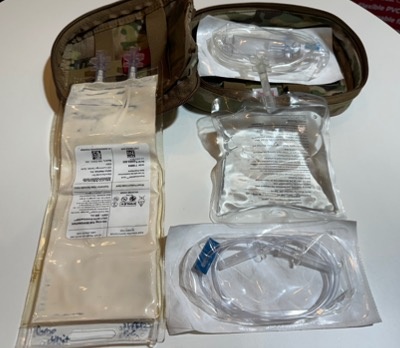
Spray dried plasma kit (source: Andrew P. Cap)
Civilian and Military Cooperation
Participants agreed that a paradigm shift is being observed across the EU towards a whole of government approach. In the past military and civilian blood establishments had very narrow interactions but the Russian invasion of Ukraine has pushed conversations to become much more in-depth and practical rather than focused on theoretical concepts.
Nevertheless, participants noted a key difference in the blood management philosophies of the civilian and military stakeholders. While blood banking at the civilian level tends to work just-in-time, the military works on the principle of just-in-case. To ensure resilience, these concepts will need to merge, and civilian stakeholders need to get used to having more redundancy in the system even though the idea of ‘wasting’ blood can be unpalatable.
The complexities of maintaining surge capacities in case of large-scale combat operations and the associated costs were also underscored. It was noted that building and maintaining these capacities requires significant funding, strategic planning and civil-military coordination to understand limitations.
Innovation and Innovative Products
Participants discussed the role that innovation in blood products can play in solving supply chain and storage challenges, particularly in contested environments. Although the best outcomes have been seen with the use of whole blood, the use of labile blood components (red blood cells, plasma, platelets) and freeze-dried plasma (FDP) still significantly improve outcomes and solve many of the problems related to readiness. Furthermore, speakers agreed that although the acquisition cost of FDP may be considered high, this is significantly outweighed by the benefits in terms of timing and availability and patient benefits (and the timeliness of said patient benefits).
Nevertheless, participants agreed that the stockpiling of medical devices such as blood bag sets, whole blood collection and processing equipment; apheresis sets, pathogen reduction systems, and testing and processing equipment was a priority as their storage is far simpler than labile blood products and the war in Ukraine has showcased that in times of crisis blood donations also increase.
Funding was mentioned again as crucial for the development of innovative products, as well as to support national blood centres to invest in logistics and harmonising processes.
Conclusion
The roundtable underscored the importance of cooperative efforts in standardisation, the merging of the civilian and military blood banking philosophies, and the importance of funding for national planning and innovation. The participants agreed on continuing to bring military and civilian stakeholders together to ensure the EU’s crisis preparedness.
Way ahead / direction
Attendees agreed that the issues discussed have become more pressing in the context of the Russian invasion of Ukraine. The low level of preparedness of the EU for a mass casualty event, especially in terms of the lack of stockpiles of blood and blood collection equipment, will significantly complicate the bloc‘s crisis response.
Participants pointed to the paradigm shift around defence in the EU as a key opportunity to foster dialogue and find more sustainable solutions to these issues. This includes through shaping legislation, contributing to strategic papers and pushing for these challenges to be included within the white papers set to be developed by the new European Commissioners for Defence and Space and for Preparedness and Crisis Management.
Attendees further underlined the importance of having a defined list of products that are most suitable for different emergency circumstances that are logistically easier to manage (for example, reconstituted dried plasma).
Participants were open to the idea of subsequent roundtables to continue the discussion and discuss how best to impact the European blood policy landscape.
References available on request from the author.
Authors:
Emma Loebler, Anton Chavdarov and Peter Tulkens, FIPRA International Public Affairs, Belgium
Nigel Talboys, MSc, L2B and Blood Transfusion Association, Belgium
Brigadier general (BEL MC)(OF-6) (ret.) Erwin Dhondt, MD, MSc, SFS, MHA, DO Consultancy, Belgium
Colonel (US Army MC)(OF-5) (ret.) Andre Cap, MD, PhD, Velico Medical, USA
Contact: Peter Tulkens
FIPRA International SRL
Rue de la Loi 227
1040 Brussels, Belgium
Email: [email protected]
Date: 06/24/2025
Source: EMMS Magazine 2025











