

Article
THE ROAD TO ACCREDITATION (Part 2 / 4)
LESSONS LEARNT FROM THE FIRST PUBLIC SECTOR ISO 15189 ACCREDITED LABORATORY IN GHANA.
IMPLEMENTATION
Setting
The37 Military Hospitalis a 400 bed specialist hospital located on the Liberation road betweenKotoka International Airportand Central Accra.It is one of the largest hospitals in the Republic of Ghana after theKorle-Bu Teaching Hospital.2 It is the main clinical laboratory service provider for staff of the Ghana Armed Forces (GAF) and also provides health service to the general public. The laboratory operates the Haematology and Chemical Pathology departments in addition to two other departments; Microbiology and Serology, and a blood transfusion service.
Baseline assessment and process initiation
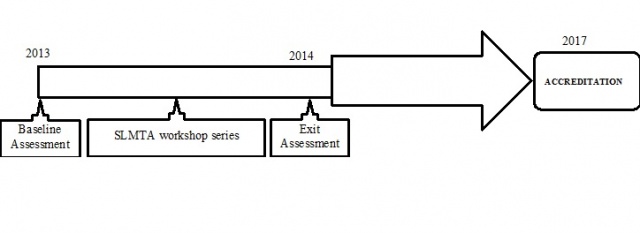
The laboratory improvement process of the Pathology Division started in February 2013 when it was selected with 5 other public sector laboratories to form the third cohort of SLMTA laboratories in Ghana. The SLMTA programme utilized the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) checklist to identify gaps and opportunities for improvement and to measure progress using a zero to five star grading system7,10. SLMTA is an innovative training and mentorship programme for continuous quality improvement with the aim of improving patient care8,10,11.
In February 2013, a baseline assessment (audit) was conducted by a team of SLMTA trained personnel from Global Health Systems Solutions (GHSS) and Ghana Health Services (GHS). This audit was conducted using the WHO-AFRO SLIPTA checklist which scores based on laboratory quality in the 12 Quality System Essentials (QSEs). Laboratories are assigned a star level based on their scores: zero star (0% - 54%), one star (55% - 64%), two stars (65% - 74%), three stars (75% - 84%), four stars (85% - 94%) and five stars (≥95%). Laboratories that score five stars are encouraged to pursue ISO 15189 accreditation.
Following the baseline assessment, four members of staff of the laboratory (laboratory manager, quality manager and 2 other laboratory staff) were selected to be trained in the SLMTA workshop series. Each of the three 5-day workshops was held three months apart to enable the laboratory implement what it had learnt from the workshops. The workshops provided the participants (who later transferred knowledge to the remaining laboratory personnel) with the requisite theoretical knowledge and practical skills to implement the requirement of the SLIPTA process and the ISO 15189. Based on the human resource capacity developed as a result of the training, laboratory management and the team developed a work plan to initiate the quality improvement process. The team led the implementation of improvement projects which included; document development, quality control, internal audits, external quality assessments and equipment management. The teams presented progress reports on the status of the projects at every laboratory management meeting.
 implementation process
implementation process
Team Formation
At the helm of affairs was the management team which had the sole responsibility of supervising and ensuring that the Laboratory Quality Management System (LQMS) was duly maintained.
To ensure the effective implementation of the process new appointments were created by Laboratory Management to ensure that specific attention is given to key aspects of the management system. These appointments included; Logistics Officers, Health and Safety Officers, Training and Development Officers and Quality Officers. These officers with the Qualitiy Manager constituted the Quality Steering Committee (QSC) and it had the sole responsibility of monitoring the Laboratory Quality Management System (LQMS), coordination and advising Laboratory Management as appropriate.
Within the life of the implementation process seven specific teams were also formed; audit team, document review team, equipment maintenance team, method validation/verification team, quality control team, external quality assessment (EQA) team and client satisfaction team. Each of these teams led by a team leader, had the responsibility of ensuring that the laboratory was meeting the set goals defined by the QSC and the LQMS.
Hospital management (Hospital Command) initiated monthly SLMTA meetings with Laboratory Management to discuss the quality improvement process and the progress to accreditation. At these meetings, the Commander of the hospital was always present for all discussions and made significant contributions geared towards the improvement of laboratory services. This represented a significant aspect of Upper Management commitment to the accreditation acquisition process.
PLEASE NOTE, the next part will be released on the 30 th March 2018.
Authors: Dr. (Col) Seth Attoh1, Mr Raymond D. Fatchu1, Mr I.T. Kodjoe1, Lt Col Richardson B Owusu1, Maj Cynthia Boateng1, Capt Alhassan M Yakubu1, Maj LX Adusu-Donkor1, Maj Joseph Boafo1, Mrs Patience N Matey1, Ms Sarah Kwao1, Mr John Ani-Amponsah1, Lt Cdr (Dr) Emmanuel Sarkodie1, Maj Kwasi Boaheng1, Flt/Lt Mary McAddy1, Capt Richmond Kyei1, Maj Monica Asante Addo1, Mr Anthony Mason1, Mr Joseph Addy1, Maj Kingsley Ackah1, Maj Anita Adjabu1, Lt Col (Dr) Fred Hobenu, Sgt Godwin Dutsyo1, Mr Godwin Nyarko1, Capt Nana Yaa F Snyper1, Capt Adu Ahenkan Poku1, Capt Christina Banamwin1, Prof Andrew Adjei2, Dr Amma Benneh-Akwasi Kuma2, Richard Asmah3, Marian Kpakpah3
Author’s affiliations: 1Pathology Division-Laboratory 37 Military Hospital, Accra-Ghana, 2College of Health Sciences University of Ghana, 3School of Allied Health Sciences- College of Health Sciences University of Ghana
Date: 03/23/2018










